Abstract
Purpose
The prognosis for high-grade glioma (HGG) patients is poor; thus, treatment-related side effects need to be minimized to conserve quality of life and functionality. Advanced techniques such as proton radiation therapy (PRT) and volumetric-modulated arc therapy (VMAT) may potentially further reduce the frequency and severity of radiogenic impairment.
Materials and methods
We retrospectively assessed 12 HGG patients who had undergone postoperative intensity-modulated proton therapy (IMPT). VMAT and 3D conformal radiotherapy (3D-CRT) plans were generated and optimized for comparison after contouring crucial neuronal structures important for neurogenesis and neurocognitive function. Integral dose (ID), homogeneity index (HI), and inhomogeneity coefficient (IC) were calculated from dose statistics. Toxicity data were evaluated.
Results
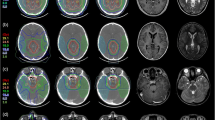
Target volume coverage was comparable for all three modalities. Compared to 3D-CRT and VMAT, PRT showed statistically significant reductions (p < 0.05) in mean dose to whole brain (−20.2 %, −22.7 %); supratentorial (−14.2 %, −20,8 %) and infratentorial (−91.0 %, −77.0 %) regions; brainstem (−67.6 %, −28.1 %); pituitary gland (−52.9 %, −52.5 %); contralateral hippocampus (−98.9 %, −98.7 %); and contralateral subventricular zone (−62.7 %, −66.7 %, respectively). Fatigue (91.7 %), radiation dermatitis (75.0 %), focal alopecia (100.0 %), nausea (41.7 %), cephalgia (58.3 %), and transient cerebral edema (16.7 %) were the most common acute toxicities.
Conclusion
Essential dose reduction while maintaining equal target volume coverage was observed using PRT, particularly in contralaterally located critical neuronal structures, areas of neurogenesis, and structures of neurocognitive functions. These findings were supported by preliminary clinical results confirming the safety and feasibility of PRT in HGG.
Zusammenfassung
Zielsetzung
Die Prognose bei „High-grade“-Gliomen (HGG) ist infaust. Gerade bei diesen Patienten sollten therapieassoziierte Nebenwirkungen minimiert werden, um die Lebensqualität und Funktionalität zu erhalten. Moderne Radiotherapietechniken, wie die Protonenradiotherapie (PRT) und die volumenmodulierte Arc-Therapie (VMAT), haben das Potential, die Dosisbelastung von Risikoorganen weiter zu reduzieren.
Material und Methoden
12 HGG-Patienten, die eine postoperative intensitätsmodulierten Protonentherapie (IMPT) erhalten hatten, wurden retrospketiv bewertet. Zum Vergleich wurden VMAT- und 3D-konformale Radiotherapiepläne (3D-CRT) generiert, in denen die Dosisverteilung in wichtigen Arealen der Neurogenese und neurokognitiven Funktion bestimmt wurden. Anhand von Dosisstatistiken wurden die Integraldosis (ID), der Homogenitätsindex (HI) und der Inhomogenitätskoeffizient (IC) berechnet und die therapieassoziierte Toxizität bestimmt.
Ergebnisse
Für alle drei Techniken war die Zielvolumenabdeckung vergleichbar gut. PRT reduzierte die Dmean im Vergleich zur 3D-CRT und VMAT im Ganzhirn (−20,2 %; −22,7 %), im supratentoriellen (−14,2 %; −20,8 %) und infratentoriellen Hirn (−91 %; −77,0 %), im Hirnstamm (−67,6 %; −28, %), in der Hypophyse (−52,9 %; −52,5 %), im kontralateralen Hippokampus (−98,9 %; −98,7 %) und in der kontralateralen subventrikulären Zone (−62,7 %; −66,7 %) signifikant (p < 0,05). Die häufigsten akuten Nebenwirkungen waren Fatigue (91,7 %), radiogene Dermatitis (75,0 %), fokale Alopezie (100,0 %), Nausea (41,7 %), Cephalgien (58,3 %) und vorübergehende zerebrale Ödeme (16,7 %).
Schlussfolgerung
Durch die PRT konnte bei Aufrechterhaltung der Zielvolumenabdeckung eine signifikante Dosisreduktion insbesondere in kontralateralen kritischen neuronalen Strukturen sowie in essentiellen Arealen für die neurokognitiven Funktionen und Neurogenese beobachtet werden. Die vorläufigen klinischen Ergebnisse bestätigen die sichere Durchführbarkeit und Praktikabilität der PRT bei HGG.

Similar content being viewed by others
References
Adeberg S, Bostel T, Konig L, Welzel T, Debus J, Combs SE (2014) A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: a predictive factor for survival? Radiat Oncol 9:95
Navarria P, Reggiori G, Pessina F, Ascolese AM, Tomatis S, Mancosu P, Lobefalo F, Clerici E, Lopci E, Bizzi A et al (2014) Investigation on the role of integrated PET/MRI for target volume definition and radiotherapy planning in patients with high grade glioma. Radiother Oncol 112(3):425–429
MacDonald SM, Ahmad S, Kachris S, Vogds BJ, DeRouen M, Gittleman AE, DeWyngaert K, Vlachaki MT (2007) Intensity modulated radiation therapy versus three-dimensional conformal radiation therapy for the treatment of high grade glioma: A dosimetric comparison. J Appl Clin Med Phys 8(2):47–60
Douw L, Klein M, Fagel SS, Heuvel J van den, Taphoorn MJ, Aaronson NK, Postma TJ, Vandertop WP, Mooij JJ, Boerman RH et al (2009) Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: Long-term follow-up. Lancet Neurol 8(9):810–818
Klein M (2012) Neurocognitive functioning in adult WHO grade II gliomas: impact of old and new treatment modalities. Neuro-oncology 14(Suppl 4):iv17–24
R-DMD K, Timmermann B, Taylor R, Scarzello G, Plasswilm L, Paulsen F, Jeremic B, Gnekow A, Dieckmann K, Kay S et al (2003) Current and future strategies in radiotherapy of childhood low-grade Glioma of the brain. Strahlenther Onkol 179(8):509–520
Chan MD (2015) Recent technical advances and indications for radiation therapy in low-grade Glioma. Semin Radiat Oncol 25(3):189–196
Aherne NJ, Benjamin LC, Horsley PJ, Silva T, Wilcox S, Amalaseelan J, Dwyer P, Tahir AM, Hill J, Last A et al (2014) Improved outcomes with intensity modulated radiation therapy combined with temozolomide for newly diagnosed glioblastoma multiforme. Neurol Res Int 2014:1–5
Shaffer R, Nichol AM, Vollans E, Fong M, Nakano S, Moiseenko V, Schmuland M, Ma R, McKenzie M, Otto K (2010) A comparison of volumetric modulated arc therapy and conventional intensity-modulated radiotherapy for frontal and temporal high-grade gliomas. Int J Radiat Oncol Biol Phys 76(4):1177–1184
Eaton BR, Yock T (2014) The use of proton therapy in the treatment of benign or low-grade pediatric brain tumors. Cancer J 20(6):5
Barani IJ, Cuttino LW, Benedict SH, Todor D, Bump EA, Wu Y, Chung TD, Broaddus WC, Lin PS (2007) Neural stem cell-preserving external-beam radiotherapy of central nervous system malignancies. Int J Radiat Oncol Biol Phys 68(4):978–985
Chera BS, Amdur RJ, Patel P, Mendenhall WM (2009) A radiation oncologist’s guide to contouring the hippocampus. Am J Clin Oncol 32(1):20–22
Merchant TE, Goloubeva O, Pritchard DL, Gaber MW, Xiong X, Danish RK, Lustig RH (2002) Radiation dose-volume effects on growth hormone secretion. Int J Radiat Oncol Biol Phys 52(5):1264–1270
Lemaire JJ, Sakka L, Ouchchane L, Caire F, Gabrillargues J, Bonny JM (2010) Anatomy of the human thalamus based on spontaneous contrast and microscopic voxels in high-field magnetic resonance imaging. Neurosurgery 66(3 Suppl Operative):161–172
Scoccianti S, Detti B, Gadda D, Greto D, Furfaro I, Meacci F, Simontacchi G, Di Brina L, Bonomo P, Giacomelli I et al (2015) Organs at risk in the brain and their dose-constraints in adults and in children: a radiation oncologist’s guide for delineation in everyday practice. Radiother Oncol 114(2):230–238
Bentzen SM, Constine LS, Deasy JO, Eisbruch A, Jackson A, Marks LB, Ten HRK, Yorke ED (2010) Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. international J Radiat Oncol Biol Phys 76(3 Suppl):3–9
Jackson A, Marks LB, Bentzen SM, Eisbruch A, Yorke ED, Ten HRK, Constine LS, Deasy JO (2010) The lessons of QUANTEC: recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys 76(3 Suppl):155–160
Marks LB, Ten HRK, Martel MK (2010) Guest editor’s introduction to QUANTEC: A users guide. Int J Radiat Oncol Biol Phys 76:1–2
Kessel KA, Bohn C, Engelmann U, Oetzel D, Bougatf N, Bendl R, Debus J, Combs SE (2014) Five-year experience with setup and implementation of an integrated database system for clinical documentation and research. Comput Methods Programs Biomed 114(2):206–217
Bougatf N, Bendl R, Debus J (2015) Towards secondary use of heterogeneous radio-oncological data for retrospective clinical trials: service-oriented connection of a central research database with image analysis tools. Proc SPIE 9418, Medical Imaging 2015: PACS and Imaging Informatics: Next generation and inoovations, 941807 (March 17, 2015). doi:10.1117/12.2084365
Bendel R (2006) Virtual therapy simultation. In: Schlegel W, Bortfeld T, and Grosu A-L (eds) New Technologies in Radiation Oncology. Springer, Berlin, pp 179–186
Kataria T, Sharma K, Subramani V, Karrthick KP, Bisht SS (2012) Homogeneity Index: An objective tool for assessment of conformal radiation treatments. J Med Phys 37(4):207–213
Claus F, Mijnheer B, Rasch C, Bortfeld T, Fraass B, De Gersem W, Wirtz H, Hoinkis C, Cho BC, Kwong LW et al (2002) Report of a study on IMRT planning strategies for ethmoid sinus cancer. Strahlenther Onkol 178(10):572–576
D’Souza WD, Rosen II (2003) Nontumor integral dose variation in conventional radiotherapy treatment planning. Med Phys 30(8):2065–2071
Fuss M, Hug EB, Schaefer RA, Nevinny-Stickel M, Miller DW, Slater JM, Slater JD (1999) Proton radiation therapy (prt) for pediatric optic pathway gliomas: comparison with 3d planned conventional photons and a standard photon technique. Int J Radiat Oncol Biol Phys 45(5):1117–1126
Darzy KH, Shalet SM (2009) Hypopituitarism following radiotherapy. Pituitary 12(1):40–50
Taphoorn MJ, Heimans JJ, Veen EA van der, Karim AB (1995) Endocrine functions in long-term survivors of low-grade supratentorial glioma treated with radiation therapy. J Neurooncol 25(2):97–102
Boehling NS, Grosshans DR, Bluett JB, Palmer MT, Song X, Amos RA, Sahoo N, Meyer JJ, Mahajan A, Woo SY (2012) Dosimetric comparison of three-dimensional Conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric Craniopharyngiomas. Int J Radiat Oncol Biol Phys 82(2):643–652
Karunamuni R, Bartsch H, White NS, Moiseenko V, Carmona R, Marshall DC, Seibert TM, McDonald CR, Farid N, Krishnan A et al (2016) Dose-dependent cortical thinning after partial brain irradiation in high-grade Glioma. Int J Radiat Oncol Biol Phys 94(2):297–304
Laack NN, Brown PD, Ivnik RJ, Furth AF, Ballman KV, Hammack JE, Arusell RM, Shaw EG, Buckner JC, North Central Cancer Treatment G (2005) Cognitive function after radiotherapy for supratentorial low-grade glioma: A north central cancer treatment group prospective study. Int J Radiat Oncol Biol Phys 63(4):1175–1183
Scott JN, Rewcastle NB, Brasher PM, Fulton D, Hagen NA, MacKinnon JA, Sutherland G, Cairncross JG, Forsyth P (1998) Long-term glioblastoma multiforme survivors: A population-based study. Can J Neurol Sci 25(3):197–201
Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U (2008) Proton versus photon radiotherapy for common pediatric brain tumors: Comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer 51(1):110–117
Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X (2009) Late effects of Conformal radiation therapy for pediatric patients with low-grade Glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol 27(22):3691–3697
Armstrong GT (2010) Long-term survivors of childhood central nervous system malignancies: The experience of the childhood cancer survivor study. Eur J Paediatr Neurol 14(4):298–303
Jalali R, Mallick I, Dutta D, Goswami S, Gupta T, Munshi A, Deshpande D, Sarin R (2010) Factors influencing Neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int J Radiat Oncol Biol Phys 77(4):974–979
Kazda T, Jancalek R, Pospisil P, Sevela O, Prochazka T, Vrzal M, Burkon P, Slavik M, Hynkova L, Slampa P et al (2014) Why and how to spare the hippocampus during brain radiotherapy: The developing role of hippocampal avoidance in cranial radiotherapy. Radiat Oncol 9:139–139
Kut C, Janson RK (2014) New considerations in radiation treatment planning for brain tumors: neural progenitor cell – containing niches. Semin Radiat Oncol 24(4):265–272
Goings GE, Sahni V, Szele FG (2004) Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res 996(2):213–226
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8(9):963–970
Redmond KJ, Mahone EM, Terezakis S, Ishaq O, Ford E, McNutt T, Kleinberg L, Cohen KJ, Wharam M, Horska A (2013) Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: a prospective study. Neuro-oncology 15(3):360–369
Mu X, Bjork-Eriksson T, Nill S, Oelfke U, Johansson KA, Gagliardi G, Johansson L, Karlsson M, Zackrisson DB (2005) Does electron and proton therapy reduce the risk of radiation induced cancer after spinal irradiation for childhood medulloblastoma? A comparative treatment planning study. Acta Oncol 44(6):554–562
Miralbell R, Lomax A, Cella L, Schneider U (2002) Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys 54(3):824–829
Narayana A, Yamada J, Berry S, Shah P, Hunt M, Gutin PH, Leibel SA (2006) Intensity-modulated radiotherapy in high-grade gliomas: Clinical and dosimetric results. Int J Radiat Oncol Biol Phys 64(3):892–897
Marsh JC, Ziel GE, Diaz AZ, Wendt JA, Gobole R, Turian JV (2013) Integral dose delivered to normal brain with conventional intensity-modulated radiotherapy (IMRT) and helical tomotherapy IMRT during partial brain radiotherapy for high-grade gliomas with and without selective sparing of the hippocampus, limbic circuit and neural stem cell compartment. J Med Imaging Radiat Oncol 57(3):378–383
Acknowledgements
We thank Eric Tonndorf-Martini and Thomas Mielke for excellent technical assistance. We acknowledge financial support of the Dietmar-Hopp-Stiftung.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Adeberg, S.B. Harrabi, N. Bougatf, D. Bernhardt, J. Rieber, S. A. Koerber, M. Syed, T. Sprave, A. Mohr, A. Abdollahi, T. Haberer, S. E. Combs, K. Herfarth, J. Debus, and S. Rieken state that there are no conflicts of interest.
Ethical standards
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form).
Rights and permissions
About this article
Cite this article
Adeberg, S., Harrabi, S.B., Bougatf, N. et al. Intensity-modulated proton therapy, volumetric-modulated arc therapy, and 3D conformal radiotherapy in anaplastic astrocytoma and glioblastoma. Strahlenther Onkol 192, 770–779 (2016). https://doi.org/10.1007/s00066-016-1007-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-016-1007-7