Abstract
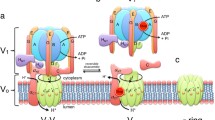
V-ATPases are multimeric enzymes made of two sectors, a V1 catalytic domain and a V0 membrane domain. They accumulate protons in various intracellular organelles. Acidification of synaptic vesicles by V-ATPase energizes the accumulation of neurotransmitters in these storage organelles and is therefore required for efficient synaptic transmission. In addition to this well-accepted role, functional studies have unraveled additional hidden roles of V0 in neurotransmitter exocytosis that are independent of the transport of protons. V0 interacts with SNAREs and calmodulin, and perturbing these interactions affects neurotransmitter release. Here, we discuss these data in relation with previous results obtained in reconstituted membranes and on yeast vacuole fusion. We propose that V0 could be a sensor of intra-vesicular pH that controls the exocytotic machinery, probably regulating SNARE complex assembly during the synaptic vesicle priming step, and that, during the membrane fusion step, V0 might favor lipid mixing and fusion pore stability.



Similar content being viewed by others
Abbreviations
- V-ATPase:
-
Vacuolar-type H+ATPase
- V0:
-
V-ATPase membrane domain
- V1:
-
V-ATPase catalytic domain
- ACh:
-
Acetylcholine
- SNARE:
-
Soluble NSF attachment receptor proteins
- CALI:
-
Chromophore-assisted light inactivation
- ARNO:
-
ADP ribosylation factor nucleotide-binding site opener
- Arf6:
-
ADP-ribosylation factor-6
References
Forgac M (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8:917–929
Toei M, Saum R, Forgac M (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49:4715–4723
Ma B, Xiang Y, An L (2011) Structural bases of physiological functions and roles of the vacuolar H+ATPase. Cell Signal 23:1244–1256
Marshansky V, Rubinstein JL, Grüber G (2014) Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta 1837:857–879
Maxson ME, Grinstein S (2014) The vacuolar-type H+-ATPase at a glance—more than a proton pump. J Cell Sci 127:4987–4993
Edwards RH (2007) The neurotransmitter cycle and quantal size. Neuron 55:835–858
Zhang Z, Zheng Y, Mazon H, Milgrom E, Kitagawa N, Kish-Trier E, Heck AJR, Kane PM, Wilkens S (2008) Structure of the yeast vacuolar ATPase. J Biol Chem 283:35983–35995
Kawasaki-Nishi S, Bowers K, Nishi T, Forgac M, Stevens TH (2001) The amino-terminal domain of the vacuolar proton- translocating ATPase a subunit controls targeting and in vivo dissociation, and the carboxyl-terminal domain affects coupling of proton transport and ATP hydrolysis. J Biol Chem 276:47411–47420
Sun-Wada G-H, Wada Y (2010) Vacuolar-type proton pump ATPases: roles of subunit isoforms in physiology and pathology. Histol Histopathol 25:1611–1620
Perin MS, Fried VA, Stone DK, Xie X-S, Südhof TC (1991) Structure of the 116-kDa polypeptide of the clathrin-coated vesicle/synaptic vesicle proton pump. J Biol Chem 266:3877–3881
Poëa-Guyon S, Amar M, Fossier P, Morel N (2006) Alternative splicing controls neuronal expression of v-ATPase subunit a1and sorting to nerve terminals. J Biol Chem 281:17164–17172
Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada G-H, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8:124–136
Saw NMN, Kang S-YA, Parsaud L, Han GA, Jiang T, Grzegorczyk K, Surkont M, Sun-Wada G-H, Wada Y, Li L, Sugita S (2011) Vacuolar H+-ATPase subunits Voa1 and Voa2 cooperatively regulate secretory vesicle acidification, transmitter uptake, and storage. Mol Biol Cell 22:3394–3409
Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada G-H, Wada Y, Futai M (2003) From lysosomes to the plasma membrane: localization of vacuolar type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem 278:22023–22030
Brown D, Paunescu TG, Breton S, Marshansky V (2009) Regulation of the V-ATPase in kidney epithelial cells: dual role in acid–base homeostasis and vesicle trafficking. J Exp Biol 212:1762–1772
Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson A-K, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, Villa A (2000) Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 25:343–346
Kornak U, Schulz A, Friedrich W, Uhlhaas S, Kremens B, Voit T, Hasan C, Bode U, Jentsch T, Kubisch C (2000) Mutations in the a3 subunit of the vacuolar H+-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet 9:2059–2063
Smith A, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE (2000) Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26:71–75
Kornak U, Reynders E, Dimopoulou A, van Reeuwijk J, Fischer B, Rajab A, Budde B, Nürnberg P, Foulquier F, the ARCL Debré-type Study Group, Lefeber D, Urban Z, Gruenewald S, Annaert W, Brunner HG, van Bokhoven H, Wevers R, Morava E, Matthijs G, Van Maldergem L, Mundlos S (2008) Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat Genet 40:32–34
Hucthagowder V, Morava E, Kornak U, Lefeber DJ, Fischer B, Dimopoulou A, Aldinger A, Choi J, Davis EC, Abuelo DN, Adamowicz M, Al-Aama J, Basel-Vanagaite L, Fernandez B, Greally MT, Gillessen-Kaesbach G, Kayserili H, Lemyre E, Tekin M, Türkmen S, Tuysuz B, Yüksel-Konuk B, Mundlos S, Van Maldergem L, Wevers RA, Urban Z (2009) Loss-of-function mutations in ATP6V0A2 impair vesicular trafficking, tropoelastin secretion and cell survival. Hum Mol Genet 18:2149–2165
Stadler H, Tsukita S (1984) Synaptic vesicles contain an ATP dependent proton pump and show ‘knob-like’ protrusions on their surface. EMBO J 3:3333–3337
Yamagata SK, Parsons SM (1989) Cholinergic synaptic vesicles contain a V-type and a P-type ATPase. J Neurochem 53:1354–1362
Michaelson DM, Angel I (1980) Determination of delta pH in cholinergic synaptic vesicles: its effect on storage and release of acetylcholine. Life Sci 27:39–44
Füldner HH, Stadler H (1982) 31P-NMR analysis of synaptic vesicles. Status of ATP and internal pH. Eur J Biochem 121:519–524
Miesenböck G, De Angelis DA, Rothman JE (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394:192–195
Morel N, Dedieu J-C, Philippe J-M (2003) Specific sorting of the a1 isoform of the V-H+ATPase a subunit to nerve terminals where it associates with both synaptic vesicles and the presynaptic plasma membrane. J Cell Sci 116:4751–4762
Nishi T, Forgac M (2000) Molecular cloning and expression of three isoforms of the 100-kDa a subunit of the mouse vacuolar proton-translocating ATPase. J Biol Chem 275:6824–6830
Peng S-B, Crider BP, Xie XS, Stone DK (1994) Alternative mRNA splicing generates tissue-specific isoforms of 116-kDa polypeptide of vacuolar proton pump. J Biol Chem 269:17262–17266
Poëa-Guyon S, Ammar MR, Erard M, Amar M, Moreau AW, Fossier P, Gleize V, Vitale N, Morel N (2013) The V-ATPase membrane domain is a sensor of granular pH that controls the exocytotic machinery. J Cell Biol 203:283–298
Morel N, Gérard V, Shiff G (1998) Vacuolar H+-ATPase domains are transported separately in axons and assemble in Torpedo nerve endings. J Neurochem 71:1702–1708
Einhorn Z, Trapani JG, Liu Q, Nicolson T (2012) Rabconnectin3α promotes stable activity of the H+ pump on synaptic vesicles in hair cells. J Neurosci 32:11144–11156
Smardon AM, Diab HI, Tarsio M, Diakov TT, Dehdar Nasab D, West RW, Kane PM (2014) The RAVE complex is an isoform-specific V-ATPase assembly factor in yeast. Mol Biol Cell 25:356–367
Dolezal V, Sbia M, Diebler M-F, Varoqui H, Morel N (1993) Effect of N, N′-dicyclohexylcarbodiimide on compartmentation and release of newly synthesized and preformed acetylcholine in Torpedo synaptosomes. J Neurochem 61:1454–1460
Michaelson DM, Burstein M, Licht R (1986) Translocation of cytosolic acetylcholine into synaptic vesicles and demonstration of vesicular release. J Biol Chem 261:6831–6835
Marshall IG, Parsons SM (1987) The vesicular acetylcholine transport system. Trends Neurosci 10:174–177
Egashira Y, Takase M, Takamori S (2015) Monitoring of vacuolar-type H+ATPase-mediated proton influx into synaptic vesicles. J Neurosci 35:3701–3710
Hori T, Takahashi T (2012) Kinetics of synaptic vesicle refilling with neurotransmitter glutamate. Neuron 76:511–517
Hicks BW, Parsons SM (1992) Characterization of the P-type and V-type ATPases of cholinergic synaptic vesicles and coupling of nucleotide hydrolysis to acetylcholine transport. J Neurochem 58:1211–1220
Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Ramner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klinghauf J, Grubmüller H, Heuser J, Wieland F, Jahn R (2006) Molecular anatomy of a trafficking organelle. Cell 127:831–846
Couteaux R, Pécot-Dechavassine M (1974) Les zones spécialisées des membranes présynaptiques. C R Acad Sci Hebd Seances Acad Sci D 278:291–293
Südhof TC (2012) The presynaptic active zone. Neuron 75:11–25
Llinas R, Sugimori M, Silver RB (1992) Microdomains of high calcium concentration in a pre-synaptic terminal. Science 256:677–679
Eggermann E, Bucurenciu I, Goswami SP, Jonas P (2012) Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nature Rev Neurosci 13:7–21
Wickner W, Schekman R (2008) Membrane fusion. Nat Struct Mol Biol 15:658–664
Sørensen JB (2009) Conflicting views on the membrane fusion machinery and the fusion pore. Annu Rev Cell Dev Biol 25:513–537
Südhof TC, Rothman JE (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323:474–477
Jahn R, Fasshauer D (2012) Molecular machines governing exocytosis of synaptic vesicles. Nature 490:201–207
Südhof TC (2013) Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80:675–690
Rizzoli SO, Betz WJ (2005) Synaptic vesicle pools. Nat Rev Neurosci 6:57–69
Imig C, Min S-W, Krinner S, Arancillo M, Rosenmund C, Südhof TC, Rhee JS, Brose N, Cooper BH (2014) The morphological and molecular nature of synaptic vesicle priming at presynaptic active zones. Neuron 84:416–431
Maximov A, Tang J, Yang X, Pang ZP, Südhof TC (2009) Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323:516–521
Park H, Li Y, Tsien RW (2012) Influence of synaptic vesicle position on release probability and exocytotic fusion mode. Science 335:1362–1366
Alabi AA, Tsien RW (2013) Perspectives on kiss-and-run: role in exocytosis, endocytosis, and neurotransmission. Annu Rev Physiol 75:393–422
He L, Wu L-G (2007) The debate on the kiss-and-run fusion at synapses. Trends Neurosci 30:447–455
Jackson MB, Chapman ER (2008) The fusion pore of Ca++-triggered exocytosis. Nat Struct Mol Biol 15:684–689
Mohrmann R, de Wit H, Verhage M, Neher E, Sørensen JB (2010) Fast vesicle fusion in living cells requires at least three SNARE complexes. Science 330:502–505
Sinha R, Ahmed S, Jahn R, Klinghauf J (2011) Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proc Nat Acad Sci USA 108:14318–14323
Shi L, Shen Q-T, Kiel A, Wang J, Wang H-W, Melia TJ, Rothman JE, Pincet F (2012) SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science 335:1355–1359
Zhou P, Bacaj T, Yang X, Pang ZP, Südhof TC (2013) Lipid-anchored SNARE lacking transmembrane regions support membrane fusion during neurotransmitter release. Neuron 80:470–483
Ramirez DM, Khvotchev M, Trauterman B, Kavalali ET (2012) Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron 73:121–134
Ramirez DM, Kavalali ET (2011) Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol 21:275–282
Melom JE, Akbergenova Y, Gavornik JP, Littleton JT (2013) Spontaneous and evoked release are independently regulated at individual active zones. J Neurosci 33:17253–17263
Hiesinger PR, Fayyazuddin A, Mehta SQ, Rosenmund T, Schulze KL, Zhai RG, Verstreken P, Cao Y, Zhou Y, Kunz J, Bellen HJ (2005) The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121:607–620
Wang D, Epstein D, Khalaf O, Srinivasan S, Williamson WR, Fayyazuddin A, Quiocho FA, Hiesinger PR (2014) Ca2+–calmodulin regulates SNARE assembly and spontaneous neurotransmitter release via v-ATPase subunit V0a1. J Cell Biol 205:21–31
Kawasaki-Nishi S, Nishi T, Forgac M (2001) Arg-735 of the 100-kDa subunit a of the yeast V-ATPase is essential for proton translocation. Proc Natl Acad Sci USA 98:12397–12402
Williamson WR, Wang D, Haberman AS, Hiesinger PR (2010) A dual function of V0-ATPase a1 provides an endolysosomal degradation mechanism in Drosophila melanogaster photoreceptors. J Cell Biol 189:885–899
Bayer MJ, Reese C, Bühler S, Peters C, Mayer A (2003) Vacuole membrane fusion: V0 functions after trans–SNARE pairing and is coupled to the Ca2+-releasing channel. J Cell Biol 162:211–222
Strasser B, Iwaszkiewicz J, Michielin O, Mayer A (2011) The V-ATPase proteolipid cylinder promotes the lipid-mixing stage of SNARE-dependent fusion of yeast vacuoles. EMBO J 30:4126–4141
Liégeois S, Benedetto A, Garnier JM, Schwab Y, Labouesse M (2006) The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J Cell Biol 173:949–961
Sun-Wada G-H, Toyomura T, Murata Y, Yamamoto A, Futai M, Wada Y (2006) The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J Cell Sci 119:4531–4540
Peri F, Nüsslein-Volhard C (2008) Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133:916–927
Camacho M, Machado JD, Montesinos MS, Criado M, Borges R (2006) Intragranular pH rapidly modulates exocytosis in adrenal chromaffin cells. J Neurochem 96:324–334
Morel N, Dunant Y, Israël M (2001) Neurotransmitter release through the V0 sector of V-ATPase. J Neurochem 79:485–488
El Far O, Seagar M (2011) A role for V-ATPase subunits in synaptic vesicle fusion ? J Neurochem 117:603–612
Israël M, Morel N, Lesbats B, Birman S, Manaranche R (1986) Purification of a pre-synaptic membrane protein that mediates a calcium-dependent translocation of acetylcholine. Proc Natl Acad Sci USA 83:9226–9230
Israël M, Meunier F-M, Morel N, Lesbats B (1987) Calcium-induced desensitization of acetylcholine release from synaptosomes or proteoliposomes equipped with mediatophore, a presynaptic membrane protein. J Neurochem 49:975–982
Birman S, Meunier F-M, Lesbats B, Le Caer JP, Rossier J, Israël M (1990) A 15-kDa proteolipid found in mediatophore preparations from Torpedo electric organ presents high sequence homology with the bovine chromaffin granule protonophore. FEBS Lett 261:303–306
Peters C, Bayer MJ, Bühler S, Andersen JS, Mann M, Mayer A (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409:581–588
Falk-Vairant J, Corrèges P, Eder-Colli L, Salem N, Roulet E, Bloc A, Meunier F-M, Lesbats B, Loctin F, Synguelakis M, Israël M, Dunant Y (1996) Quantal acetylcholine release induced by mediatophore transfection. Proc Natl Acad Sci USA 93:5203–5207
Bloc A, Bugnard E, Dunant Y, Falk-Vairant J, Israël M, Loctin F, Roulet E (1999) Acetylcholine synthesis and quantal release reconstituted by transfection of mediatophore and choline acetyltranferase cDNAs. Eur J Neurosci 11:1523–1534
Cavalli A, Dunant Y, Leroy C, Meunier F-M, Morel N, Israël M (1993) Antisense probes against mediatophore block transmitter release in oocytes primed with neuronal mRNAs. Eur J Neurosci 5:1539–1544
Holzenburg A, Jones PC, Franklin T, Pal T, Heimburg T, Marsh D, Findlay JBC, Finbow ME (1993) Evidence for a common structure for a class of membrane channels. Eur J Biochem 213:21–30
Kriebel ME, Gross CE (1974) Multimodal distribution of frog miniature end-plate potentials in adult, denervated, and tadpole leg muscle. J Gen Physiol 64:85–103
Erxleben C, Kriebel ME (1988) Characteristics of spontaneous miniature and subminiature end-plate currents at the mouse neuromuscular junction. J Physiol Lond 400:645–658
Muller D, Dunant Y (1987) Spontaneous quantal and subquantal transmitter release at the torpedo nerve-electroplaque junction. Neuroscience 20:911–921
Evers J, Laser M, Sun Y-A, Xie Z-P, Poo M-M (1989) Studies of nerve-muscle interactions in Xenopus cell culture: analysis of early synaptic currents. J Neurosci 9:1523–1539
Wickner W (2010) Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol 26:115–136
Ungermann C, Sato K, Wickner W (1998) Defining the functions of trans–SNARE pairs. Nature 396:543–548
Reese C, Mayer A (2005) Transition from hemifusion to pore opening is rate limiting for vacuole membrane fusion. J Cell Biol 171:981–990
Collins KM, Wickner WT (2007) Trans–SNARE complex assembly and yeast vacuole membrane fusion. Proc Natl Acad Sci USA 104:8755–8760
Manolson MF, Wu B, Proteau D, Taillon BE, Roberts BT, Hoyt MA, Jones EW (1994) STVl gene encodes functional homologue of 95-kDa yeast vacuolar H+-ATPase subunit Vphlp. J Biol Chem 269:14064–14074
Baars TL, Petri S, Peters C, Mayer A (2007) Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium. Mol Biol Cell 18:3873–3882
Takeda K, Cabrera M, Rohde J, Bausch D, Jensen ON, Ungermann C (2008) The vacuolar V1/V0-ATPase is involved in the release of the HOPS subunit Vps41 from vacuoles, vacuole fragmentation and fusion. FEBS Lett 582:1558–1563
Flannery AR, Graham LA, Stevens TH (2004) Topological characterization of the c, c’, and c″ subunits of the vacuolar ATPase from the yeast Saccharomyces cerevisiae. J Biol Chem 279:39856–39862
Coonrod EM, Graham LA, Carpp LN, Carr TM, Stirrat L, Bowers K, Bryant NJ, Stevens TH (2013) Homotypic vacuole fusion in yeast requires organelle acidification and not the V-ATPase membrane domain. Dev Cell 27:462–468
Qi J, Forgac M (2007) Cellular environment is important in controlling V-ATPase dissociation and its dependence on activity. J Biol Chem 282:24743–24751
Sreelatha A, Bennett TL, Carpinone EM, O’Brien KM, Jordan KD, Burdett DL, Orth K, Starai VJ (2015) Vibrio effector protein VopQ inhibits fusion of V-ATPase–containing membranes. Proc Natl Acad Sci USA 112:100–105
Taubenblatt P, Dedieu J-C, Gulik-Krzywicki T, Morel N (1999) VAMP (synaptobrevin) is present in the plasma membrane of nerve terminals. J Cell Sci 112:3559–3567
Granseth B, Odermatt B, Royle SJ, Lagnado L (2006) Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51:773–786
Galli T, McPherson PS, De Camilli P (1996) The V0 sector of the V-ATPase, synaptobrevin, and synaptophysin are associated on synaptic vesicles in a Triton X-100-resistant, freeze-thawing sensitive, complex. J Biol Chem 271:2193–2198
Di Giovanni J, Boudkkazi S, Mochida S, Bialowas A, Samari N, Lévêque C, Youssouf F, Brechet A, Iborra C, Maulet Y, Moutot N, Debanne D, Seagar M, El Far O (2010) V-ATPase membrane sector associates with synatobrevin to modulate neurotransmitter release. Neuron 67:268–279
Schwartz JH, Li G, Yang Q, Suri V, Ross JJ, Alexander EA (2007) Role of SNAREs and H+-ATPase in the targeting of proton pump-coated vesicles to collecting duct cell apical membrane. Kidney Int 72:1310–1315
Zhang W, Wang D, Volk E, Bellen HJ, Hiesinger PR, Quiocho FA (2008) V-ATPase V0 sector subunit a1 in neurons is a target of calmodulin. J Biol Chem 283:294–300
Lipstein N, Sakaba T, Cooper BH, Lin K-H, Strenzke N, Ashery U, Rhee J-S, Taschenberger H, Neher E, Brose N (2013) Dynamic control of synaptic vesicle replenishment and short term plasticity by Ca2+-calmodulin-Munc13-1 signaling. Neuron 79:82–96
Di Giovanni J, Iborra C, Maulet Y, Lévêque C, El Far O, Seagar M (2010) Calcium-dependent regulation of SNARE-mediated membrane fusion by calmodulin. J Biol Chem 285:23665–23675
Kuijpers GA, Rosario LM, Ornberg RL (1989) Role of intracellular pH in secretion from adrenal medulla chromaffin cells. J Biol Chem 264:698–705
Barg S, Huang P, Eliasson L, Nelson DJ, Obermüller S, Rorsman P, Thévenod F, Renström E (2001) Priming of insulin granules for exocytosis by granular Cl− uptake and acidification. J Cell Sci 114:2145–2154
Cousin MA, Nicholls DG (1997) Synaptic vesicle recycling in cultured cerebellar granule cells: role of vesicular acidification and refilling. J Neurochem 69:1927–1935
Morel N (2003) Neurotransmitter release: the dark side of the vacuolar-H+ATPase. Biol Cell 95:453–457
Hosokawa H, Dip PV, Merkulova M, Bakulina A, Zhuang Z, Khatri A, Jian X, Keating SM, Bueler SA, Rubinstein JL, Randazzo PA, Ausiello DA, Grüber G, Marshansky V (2013) The N termini of a-subunit isoforms are involved in signaling between vacuolar H+-ATPase (V-ATPase) and cytohesin-2. J Biol Chem 288:5896–5913
Béglé A, Tryoen-Toth P, de Barry J, Bader MF, Vitale N (2009) ARF6 regulates the synthesis of fusogenic lipids for calcium-regulated exocytosis in neuroendocrine cells. J Biol Chem 284:4836–4845
Vitale N, Chasserot-Golaz S, Bailly Y, Morinaga N, Frohman MA, Bader MF (2002) Calcium-regulated exocytosis of dense-core vesicles requires the activation of ADP-ribosylation factor (ARF)6 by ARF nucleotide binding site opener at the plasma membrane. J Cell Biol 159:79–89
Murata T, Yamato I, Kakinuma Y, Leslie AGW, Walker JE (2005) Structure of the rotor of the v-type Na+-ATPase from Enterococcus hirae. Science 308:654–659
Clare DK, Orlova EV, Finbow MA, Harrison MA, Findlay JBC, Saibil HR (2006) An expanded and flexible form of the vacuolar ATPase membrane sector. Structure 14:1149–1156
Bowman BJ, McCall ME, Baertsch R, Bowman EJ (2006) A model for the proteolipid ring and bafilomycin/concanamycin-binding site in the vacuolar ATPase of Neurospora crassa. J Biol Chem 281:31885–31893
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morel, N., Poëa-Guyon, S. The membrane domain of vacuolar H+ATPase: a crucial player in neurotransmitter exocytotic release. Cell. Mol. Life Sci. 72, 2561–2573 (2015). https://doi.org/10.1007/s00018-015-1886-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-1886-2