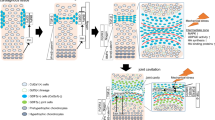
Abstract
Congenital heart defects affect approximately 1–5 % of human newborns each year, and of these cardiac defects 20–30 % are due to heart valve abnormalities. Recent literature indicates that the key factors and pathways that regulate valve development are also implicated in congenital heart defects and valve disease. Currently, there are limited options for treatment of valve disease, and therefore having a better understanding of valve development can contribute critical insight into congenital valve defects and disease. There are three major signaling pathways required for early specification and initiation of endothelial-to-mesenchymal transformation (EMT) in the cardiac cushions: BMP, TGF-β, and Notch signaling. BMPs secreted from the myocardium set up the environment for the overlying endocardium to become activated; Notch signaling initiates EMT; and both BMP and TGF-β signaling synergize with Notch to promote the transition of endothelia to mesenchyme and the mesenchymal cell invasiveness. Together, these three essential signaling pathways help form the cardiac cushions and populate them with mesenchyme and, consequently, set off the cascade of events required to develop mature heart valves. Furthermore, integration and cross-talk between these pathways generate highly stratified and delicate valve leaflets and septa of the heart. Here, we discuss BMP, TGF-β, and Notch signaling pathways during mouse cardiac cushion formation and how they together produce a coordinated EMT response in the developing mouse valves.



Similar content being viewed by others
References
Hoffman JI, Kaplan S (2002) The incidence of congenital heart disease. J Am Coll Cardiol 39(12):1890–1900
Pierpont ME, Basson CT, Benson DW Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL (2007) Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115(23):3015–3038. doi:10.1161/circulationaha.106.183056
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J (2011) Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123(4):e18–e209. doi:10.1161/CIR.0b013e3182009701
Hinton RB Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE (2006) Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 98(11):1431–1438. doi:10.1161/01.RES.0000224114.65109.4e
Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ (2001) Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 104(21):2525–2532
Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ (2004) Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis 13(5):841–847
Hinton RB, Yutzey KE (2011) Heart valve structure and function in development and disease. Annu Rev Physiol 73:29–46. doi:10.1146/annurev-physiol-012110-142145
Pomerance A (1972) Pathogenesis of aortic stenosis and its relation to age. Br Heart J 34(6):569–574
Passik CS, Ackermann DM, Pluth JR, Edwards WD (1987) Temporal changes in the causes of aortic stenosis: a surgical pathologic study of 646 cases. Mayo Clin Proc 62(2):119–123
Peterson MD, Roach RM, Edwards JE (1985) Types of aortic stenosis in surgically removed valves. Arch Pathol Lab Med 109(9):829–832
Stephan PJ, Henry AC 3rd, Hebeler RF Jr, Whiddon L, Roberts WC (1997) Comparison of age, gender, number of aortic valve cusps, concomitant coronary artery bypass grafting, and magnitude of left ventricular-systemic arterial peak systolic gradient in adults having aortic valve replacement for isolated aortic valve stenosis. Am J Cardiol 79(2):166–172
Wirrig EE, Hinton RB, Yutzey KE (2011) Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol 50(3):561–569. doi:10.1016/j.yjmcc.2010.12.005
Lincoln J, Lange AW, Yutzey KE (2006) Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol 294(2):292–302. doi:10.1016/j.ydbio.2006.03.027
Sheikh AM, Livesey SA (2010) Surgical management of valve disease in the early 21st century. Clin Med 10(2):177–181
Abu-Issa R, Kirby ML (2007) Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol 23:45–68. doi:10.1146/annurev.cellbio.23.090506.123331
Combs MD, Yutzey KE (2009) Heart valve development: regulatory networks in development and disease. Circ Res 105(5):408–421. doi:10.1161/circresaha.109.201566
Snarr BS, Kern CB, Wessels A (2008) Origin and fate of cardiac mesenchyme. Dev Dyn 237(10):2804–2819. doi:10.1002/dvdy.21725
Person AD, Klewer SE, Runyan RB (2005) Cell biology of cardiac cushion development. Int Rev Cytol 243:287–335. doi:10.1016/s0074-7696(05)43005-3
Camenisch TD, Molin DG, Person A, Runyan RB, de Groot ACG, McDonald JA, Klewer SE (2002) Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol 248(1):170–181
Schroeder JA, Jackson LF, Lee DC, Camenisch TD (2003) Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med (Berl) 81(7):392–403. doi:10.1007/s00109-003-0456-5
Markwald RR, Fitzharris TP, Manasek FJ (1977) Structural development of endocardial cushions. Am J Anat 148(1):85–119. doi:10.1002/aja.1001480108
Restivo A, Piacentini G, Placidi S, Saffirio C, Marino B (2006) Cardiac outflow tract: a review of some embryogenetic aspects of the conotruncal region of the heart. Anat Rec A Discov Mol Cell Evol Biol 288(9):936–943. doi:10.1002/ar.a.20367
Hinton RB Jr, Alfieri CM, Witt SA, Glascock BJ, Khoury PR, Benson DW, Yutzey KE (2008) Mouse heart valve structure and function: echocardiographic and morphometric analyses from the fetus through the aged adult. Am J Physiol Heart Circ Physiol 294(6):H2480–H2488. doi:10.1152/ajpheart.91431.2007
Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ (2006) Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113(10):1344–1352. doi:10.1161/circulationaha.105.591768
Butcher JT, Markwald RR (2007) Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci 362(1484):1489–1503. doi:10.1098/rstb.2007.2130
Kruithof BP, Krawitz SA, Gaussin V (2007) Atrioventricular valve development during late embryonic and postnatal stages involves condensation and extracellular matrix remodeling. Dev Biol 302(1):208–217. doi:10.1016/j.ydbio.2006.09.024
Sacks MS, Yoganathan AP (2007) Heart valve function: a biomechanical perspective. Philos Trans R Soc Lond B Biol Sci 362(1484):1369–1391. doi:10.1098/rstb.2007.2122
Vesely I (1998) The role of elastin in aortic valve mechanics. J Biomech 31(2):115–123
de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gier-de VC, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM (2004) Lineage and morphogenetic analysis of the cardiac valves. Circ Res 95(6):645–654. doi:10.1161/01.RES.0000141429.13560.cb
Lincoln J, Alfieri CM, Yutzey KE (2004) Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn 230(2):239–250. doi:10.1002/dvdy.20051
Wessels A, van den Hoff MJ, Adamo RF, Phelps AL, Lockhart MM, Sauls K, Briggs LE, Norris RA, van Wijk B, Perez-Pomares JM, Dettman RW, Burch JB (2012) Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev Biol 366(2):111–124. doi:10.1016/j.ydbio.2012.04.020
Liu AC, Joag VR, Gotlieb AI (2007) The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 171(5):1407–1418. doi:10.2353/ajpath.2007.070251
Xiao H, Zhang YY (2008) Understanding the role of transforming growth factor-beta signalling in the heart: overview of studies using genetic mouse models. Clin Exp Pharmacol Physiol 35(3):335–341. doi:10.1111/j.1440-1681.2007.04876.x
Conway SJ, Doetschman T, Azhar M (2011) The inter-relationship of periostin, TGF beta, and BMP in heart valve development and valvular heart diseases. ScientificWorldJournal 11:1509–1524. doi:10.1100/tsw.2011.132
Zhang YE (2009) Non-Smad pathways in TGF-beta signaling. Cell Res 19(1):128–139. doi:10.1038/cr.2008.328
Stevens MV, Parker P, Vaillancourt RR, Camenisch TD (2006) MEKK4 regulates developmental EMT in the embryonic heart. Dev Dyn 235(10):2761–2770. doi:10.1002/dvdy.20922
Sakabe M, Ikeda K, Nakatani K, Kawada N, Imanaka-Yoshida K, Yoshida T, Yamagishi T, Nakajima Y (2006) Rho kinases regulate endothelial invasion and migration during valvuloseptal endocardial cushion tissue formation. Dev Dyn 235(1):94–104. doi:10.1002/dvdy.20648
Feng Q, Di R, Tao F, Chang Z, Lu S, Fan W, Shan C, Li X, Yang Z (2010) PDK1 regulates vascular remodeling and promotes epithelial-mesenchymal transition in cardiac development. Mol Cell Biol 30(14):3711–3721. doi:10.1128/mcb.00420-10
Zhu HJ, Burgess AW (2001) Regulation of transforming growth factor-beta signaling. Mol Cell Biol Res Commun 4(6):321–330. doi:10.1006/mcbr.2001.0301
Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K (1997) Smad6 inhibits signalling by the TGF-beta superfamily. Nature 389(6651):622–626. doi:10.1038/39355
Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P (1997) Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 389(6651):631–635. doi:10.1038/39369
Park SH (2005) Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J Biochem Mol Biol 38(1):9–16
Ma L, Lu MF, Schwartz RJ, Martin JF (2005) Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132(24):5601–5611. doi:10.1242/dev.02156
Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL (2003) An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev 17(19):2362–2367. doi:10.1101/gad.1124803
Solloway MJ, Robertson EJ (1999) Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development 126(8):1753–1768
Kim RY, Robertson EJ, Solloway MJ (2001) Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol 235(2):449–466. doi:10.1006/dbio.2001.0284
Somi S, Buffing AA, Moorman AF, Van Den Hoff MJ (2004) Dynamic patterns of expression of BMP isoforms 2, 4, 5, 6, and 7 during chicken heart development. Anat Rec A Discov Mol Cell Evol Biol 279(1):636–651. doi:10.1002/ar.a.20031
Somi S, Buffing AA, Moorman AF, Van Den Hoff MJ (2004) Expression of bone morphogenetic protein-10 mRNA during chicken heart development. Anat Rec A Discov Mol Cell Evol Biol 279(1):579–582. doi:10.1002/ar.a.20052
Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V (2005) Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol 286(1):299–310. doi:10.1016/j.ydbio.2005.07.035
Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P (1995) Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology 136(6):2652–2663
Roelen BA, Goumans MJ, van Rooijen MA, Mummery CL (1997) Differential expression of BMP receptors in early mouse development. Int J Dev Biol 41(4):541–549
Yamagishi T, Nakajima Y, Miyazono K, Nakamura H (1999) Bone morphogenetic protein-2 acts synergistically with transforming growth factor-beta3 during endothelial-mesenchymal transformation in the developing chick heart. J Cell Physiol 180(1):35–45. doi:10.1002/(sici)1097-4652(199907)180:1<35:aid-jcp4>3.0.co;2-r
Zhang H, Bradley A (1996) Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122(10):2977–2986
Sugi Y, Yamamura H, Okagawa H, Markwald RR (2004) Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol 269(2):505–518. doi:10.1016/j.ydbio.2004.01.045
Luna-Zurita L, Prados B, Grego-Bessa J, Luxan G, del Monte G, Benguria A, Adams RH, Perez-Pomares JM, de la Pompa JL (2010) Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J Clin Invest 120(10):3493–3507. doi:10.1172/jci42666
McCulley DJ, Kang JO, Martin JF, Black BL (2008) BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn 237(11):3200–3209. doi:10.1002/dvdy.21743
Uchimura T, Komatsu Y, Tanaka M, McCann KL, Mishina Y (2009) Bmp2 and Bmp4 genetically interact to support multiple aspects of mouse development including functional heart development. Genesis 47(6):374–384. doi:10.1002/dvg.20511
Kingsley DM, Bland AE, Grubber JM, Marker PC, Russell LB, Copeland NG, Jenkins NA (1992) The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell 71(3):399–410
Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ (1998) Mice lacking Bmp6 function. Dev Genet 22(4):321–339. doi:10.1002/(sici)1520-6408(1998)22:4<321:aid-dvg3>3.0.co;2-8
Dudley AT, Lyons KM, Robertson EJ (1995) A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9(22):2795–2807
Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, Li E (1999) The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development 126(11):2551–2561
Mishina Y, Suzuki A, Ueno N, Behringer RR (1995) Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 9(24):3027–3037
Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K (2000) BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol 221(1):249–258. doi:10.1006/dbio.2000.9670
Song L, Fassler R, Mishina Y, Jiao K, Baldwin HS (2007) Essential functions of Alk3 during AV cushion morphogenesis in mouse embryonic hearts. Dev Biol 301(1):276–286. doi:10.1016/j.ydbio.2006.08.004
Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD (2002) Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci USA 99(5):2878–2883. doi:10.1073/pnas.042390499
Gaussin V, Morley GE, Cox L, Zwijsen A, Vance KM, Emile L, Tian Y, Liu J, Hong C, Myers D, Conway SJ, Depre C, Mishina Y, Behringer RR, Hanks MC, Schneider MD, Huylebroeck D, Fishman GI, Burch JB, Vatner SF (2005) Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ Res 97(3):219–226. doi:10.1161/01.res.0000177862.85474.63
Delot EC, Bahamonde ME, Zhao M, Lyons KM (2003) BMP signaling is required for septation of the outflow tract of the mammalian heart. Development 130(1):209–220
Kruithof BP, Duim SN, Moerkamp AT, Goumans MJ (2012) TGFbeta and BMP signaling in cardiac cushion formation: lessons from mice and chicken. Differentiation. doi:10.1016/j.diff.2012.04.003
Beppu H, Malhotra R, Beppu Y, Lepore JJ, Parmacek MS, Bloch KD (2009) BMP type II receptor regulates positioning of outflow tract and remodeling of atrioventricular cushion during cardiogenesis. Dev Biol 331(2):167–175. doi:10.1016/j.ydbio.2009.04.032
Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS (2001) Bone formation and inflammation in cardiac valves. Circulation 103(11):1522–1528
Kaden JJ, Bickelhaupt S, Grobholz R, Vahl CF, Hagl S, Brueckmann M, Haase KK, Dempfle CE, Borggrefe M (2004) Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis 13(4):560–566
Ankeny RF, Thourani VH, Weiss D, Vega JD, Taylor WR, Nerem RM, Jo H (2011) Preferential activation of SMAD1/5/8 on the fibrosa endothelium in calcified human aortic valves–association with low BMP antagonists and SMAD6. PLoS ONE 6(6):e20969. doi:10.1371/journal.pone.0020969
Yamagishi T, Ando K, Nakamura H (2009) Roles of TGFbeta and BMP during valvulo-septal endocardial cushion formation. Anat Sci Int 84(3):77–87. doi:10.1007/s12565-009-0027-0
Potts JD, Runyan RB (1989) Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol 134(2):392–401
Akhurst RJ, Lehnert SA, Faissner A, Duffie E (1990) TGF beta in murine morphogenetic processes: the early embryo and cardiogenesis. Development 108(4):645–656
Molin DG, Bartram U, Van der Heiden K, Van Iperen L, Speer CP, Hierck BP, Poelmann RE, Gittenberger-de-Groot AC (2003) Expression patterns of Tgfbeta1-3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton. Dev Dyn 227(3):431–444. doi:10.1002/dvdy.10314
Mariano JM, Montuenga LM, Prentice MA, Cuttitta F, Jakowlew SB (1998) Concurrent and distinct transcription and translation of transforming growth factor-beta type I and type II receptors in rodent embryogenesis. Int J Dev Biol 42(8):1125–1136
Mummery CL (2001) Transforming growth factor beta and mouse development. Microsc Res Tech 52(4):374–386. doi:10.1002/1097-0029(20010215)52:4<374:aid-jemt1022>3.0.co;2-8
Seki T, Hong KH, Oh SP (2006) Nonoverlapping expression patterns of ALK1 and ALK5 reveal distinct roles of each receptor in vascular development. Lab Invest 86(2):116–129. doi:10.1038/labinvest.3700376
Roelen BA, van Rooijen MA, Mummery CL (1997) Expression of ALK-1, a type 1 serine/threonine kinase receptor, coincides with sites of vasculogenesis and angiogenesis in early mouse development. Dev Dyn 209(4):418–430. doi:10.1002/(sici)1097-0177(199708)209:4<418:aid-aja9>3.0.co;2-l
Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, Small C, Weinberg RA, Sizeland AM, Zhu HJ (2003) Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol Cell Biol 23(12):4371–4385
Qu R, Silver MM, Letarte M (1998) Distribution of endoglin in early human development reveals high levels on endocardial cushion tissue mesenchyme during valve formation. Cell Tissue Res 292(2):333–343
Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB (1991) Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci USA 88(4):1516–1520
Ramsdell AF, Markwald RR (1997) Induction of endocardial cushion tissue in the avian heart is regulated, in part, by TGFbeta-3-mediated autocrine signaling. Dev Biol 188(1):64–74. doi:10.1006/dbio.1997.8637
Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB (1999) TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol 208(2):530–545. doi:10.1006/dbio.1999.9211
Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ (1995) Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 121(6):1845–1854
Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D et al (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359(6397):693–699. doi:10.1038/359693a0
Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB (1994) Maternal rescue of transforming growth factor-beta 1 null mice. Science 264(5167):1936–1938
Sanford LP, Ormsby I, de Groot ACG, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T (1997) TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124(13):2659–2670
Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, de Groot ACG (2001) Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation 103(22):2745–2752
Azhar M, Runyan RB, Gard C, Sanford LP, Miller ML, Andringa A, Pawlowski S, Rajan S, Doetschman T (2009) Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev Dyn 238(2):431–442. doi:10.1002/dvdy.21854
Azhar M, Brown K, Gard C, Chen H, Rajan S, Elliott DA, Stevens MV, Camenisch TD, Conway SJ, Doetschman T (2011) Transforming growth factor Beta2 is required for valve remodeling during heart development. Dev Dyn 240(9):2127–2141. doi:10.1002/dvdy.22702
Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J (1995) Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 11(4):415–421. doi:10.1038/ng1295-415
Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S (2001) Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J 20(7):1663–1673. doi:10.1093/emboj/20.7.1663
Oshima M, Oshima H, Taketo MM (1996) TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol 179(1):297–302. doi:10.1006/dbio.1996.0259
Carvalho RL, Itoh F, Goumans MJ, Lebrin F, Kato M, Takahashi S, Ema M, Itoh S, van Rooijen M, Bertolino P, Ten Dijke P, Mummery CL (2007) Compensatory signalling induced in the yolk sac vasculature by deletion of TGFbeta receptors in mice. J Cell Sci 120(Pt 24):4269–4277. doi:10.1242/jcs.013169
Sridurongrit S, Larsson J, Schwartz R, Ruiz-Lozano P, Kaartinen V (2008) Signaling via the Tgf-beta type I receptor Alk5 in heart development. Dev Biol 322(1):208–218. doi:10.1016/j.ydbio.2008.07.038
Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E (2000) Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA 97(6):2626–2631
Jiao K, Langworthy M, Batts L, Brown CB, Moses HL, Baldwin HS (2006) Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development 133(22):4585–4593. doi:10.1242/dev.02597
Robson A, Allinson KR, Anderson RH, Henderson DJ, Arthur HM (2010) The TGFbeta type II receptor plays a critical role in the endothelial cells during cardiac development. Dev Dyn 239(9):2435–2442. doi:10.1002/dvdy.22376
Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, Burn J, Diamond AG (2000) Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol 217(1):42–53. doi:10.1006/dbio.1999.9534
Bourdeau A, Dumont DJ, Letarte M (1999) A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest 104(10):1343–1351. doi:10.1172/jci8088
Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP (1999) Defective angiogenesis in mice lacking endoglin. Science 284(5419):1534–1537
Compton LA, Potash DA, Brown CB, Barnett JV (2007) Coronary vessel development is dependent on the type III transforming growth factor beta receptor. Circ Res 101(8):784–791. doi:10.1161/circresaha.107.152082
Rosenkranz S (2004) TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 63(3):423–432. doi:10.1016/j.cardiores.2004.04.030
Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA (2004) Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 95(3):253–260. doi:10.1161/01.RES.0000136520.07995.aa
Liu AC, Gotlieb AI (2008) Transforming growth factor-beta regulates in vitro heart valve repair by activated valve interstitial cells. Am J Pathol 173(5):1275–1285. doi:10.2353/ajpath.2008.080365
Jian B, Narula N, Li QY, Mohler ER 3rd, Levy RJ (2003) Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg 75(2):457–465 (discussion 465–456)
Hulin A, Deroanne CF, Lambert CA, Dumont B, Castronovo V, Defraigne JO, Nusgens BV, Radermecker MA, Colige AC (2012) Metallothionein-dependent up-regulation of TGF-beta2 participates in the remodelling of the myxomatous mitral valve. Cardiovasc Res 93(3):480–489. doi:10.1093/cvr/cvr337
Girdauskas E, Schulz S, Borger MA, Mierzwa M, Kuntze T (2011) Transforming growth factor-beta receptor type II mutation in a patient with bicuspid aortic valve disease and intraoperative aortic dissection. Ann Thorac Surg 91(5):e70–e71. doi:10.1016/j.athoracsur.2010.12.060
Attaran S, Sherwood R, Dastidar MG, El-Gamel A (2010) Identification of low circulatory transforming growth factor beta-1 in patients with degenerative heart valve disease. Interact Cardiovasc Thorac Surg 11(6):791–793. doi:10.1510/icvts.2010.244384
Goumans MJ, Mummery C (2000) Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44(3):253–265
Zhu Y, Richardson JA, Parada LF, Graff JM (1998) Smad3 mutant mice develop metastatic colorectal cancer. Cell 94(6):703–714
Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF (1999) Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol 19(4):2495–2504
Hester M, Thompson JC, Mills J, Liu Y, El-Hodiri HM, Weinstein M (2005) Smad1 and Smad8 function similarly in mammalian central nervous system development. Mol Cell Biol 25(11):4683–4692. doi:10.1128/mcb.25.11.4683-4692.2005
Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, Wynshaw-Boris A, Roberts AB (2001) Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol 240(1):157–167. doi:10.1006/dbio.2001.0469
Tremblay KD, Dunn NR, Robertson EJ (2001) Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 128(18):3609–3621
Weinstein M, Yang X, Li C, Xu X, Gotay J, Deng CX (1998) Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc Natl Acad Sci USA 95(16):9378–9383
Nomura M, Li E (1998) Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature 393(6687):786–790. doi:10.1038/31693
Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern SE, Rossant J, Mak TW (1998) The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev 12(1):107–119
Yang X, Li C, Xu X, Deng C (1998) The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci USA 95(7):3667–3672
Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A (1999) Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 126(8):1631–1642
Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA Jr, Falb D, Huszar D (2000) A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet 24(2):171–174. doi:10.1038/72835
Chen Q, Chen H, Zheng D, Kuang C, Fang H, Zou B, Zhu W, Bu G, Jin T, Wang Z, Zhang X, Chen J, Field LJ, Rubart M, Shou W, Chen Y (2009) Smad7 is required for the development and function of the heart. J Biol Chem 284(1):292–300. doi:10.1074/jbc.M807233200
Moskowitz IP, Wang J, Peterson MA, Pu WT, Mackinnon AC, Oxburgh L, Chu GC, Sarkar M, Berul C, Smoot L, Robertson EJ, Schwartz R, Seidman JG, Seidman CE (2011) Transcription factor genes Smad4 and Gata4 cooperatively regulate cardiac valve development [corrected]. Proc Natl Acad Sci USA 108(10):4006–4011. doi:10.1073/pnas.1019025108
Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 5(2):207–216. doi:S1097-2765(00)80417-7
Wen C, Metzstein MM, Greenwald I (1997) SUP-17, a Caenorhabditis elegans ADAM protein related to Drosophila KUZBANIAN, and its role in LIN-12/NOTCH signalling. Development 124(23):4759–4767
Parks AL, Klueg KM, Stout JR, Muskavitch MA (2000) Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development 127(7):1373–1385
De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R (1999) A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398(6727):518–522. doi:10.1038/19083
Okochi M, Steiner H, Fukumori A, Tanii H, Tomita T, Tanaka T, Iwatsubo T, Kudo T, Takeda M, Haass C (2002) Presenilins mediate a dual intramembranous gamma-secretase cleavage of Notch-1. EMBO J 21(20):5408–5416
Kurooka H, Kuroda K, Honjo T (1998) Roles of the ankyrin repeats and C-terminal region of the mouse notch1 intracellular region. Nucleic Acids Res 26(23):5448–5455
Beatus P, Lundkvist J, Oberg C, Pedersen K, Lendahl U (2001) The origin of the ankyrin repeat region in Notch intracellular domains is critical for regulation of HES promoter activity. Mech Dev 104(1–2):3–20
Ong CT, Cheng HT, Chang LW, Ohtsuka T, Kageyama R, Stormo GD, Kopan R (2006) Target selectivity of vertebrate notch proteins. Collaboration between discrete domains and CSL-binding site architecture determines activation probability. J Biol Chem 281(8):5106–5119. doi:10.1074/jbc.M506108200
Hsieh JJ, Henkel T, Salmon P, Robey E, Peterson MG, Hayward SD (1996) Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol Cell Biol 16(3):952–959
Wu L, Sun T, Kobayashi K, Gao P, Griffin JD (2002) Identification of a family of mastermind-like transcriptional coactivators for mammalian notch receptors. Mol Cell Biol 22(21):7688–7700
Davis RL, Turner DL (2001) Vertebrate hairy and Enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene 20(58):8342–8357. doi:10.1038/sj.onc.1205094
Iso T, Chung G, Hamamori Y, Kedes L (2002) HERP1 is a cell type-specific primary target of Notch. J Biol Chem 277(8):6598–6607. doi:10.1074/jbc.M110495200
Noseda M, Fu Y, Niessen K, Wong F, Chang L, McLean G, Karsan A (2006) Smooth Muscle alpha-actin is a direct target of Notch/CSL. Circ Res 98(12):1468–1470. doi:10.1161/01.res.0000229683.81357.26
Niessen K, Fu Y, Chang L, Hoodless PA, McFadden D, Karsan A (2008) Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J Cell Biol 182(2):315–325. doi:10.1083/jcb.200710067
Fu Y, Chang A, Chang L, Niessen K, Eapen S, Setiadi A, Karsan A (2009) Differential regulation of transforming growth factor beta signaling pathways by Notch in human endothelial cells. J Biol Chem 284(29):19452–19462. doi:10.1074/jbc.M109.011833
Fu Y, Chang AC, Fournier M, Chang L, Niessen K, Karsan A (2011) RUNX3 maintains the mesenchymal phenotype after termination of the Notch signal. J Biol Chem 286(13):11803–11813. doi:10.1074/jbc.M111.222331
Andersson ER, Sandberg R, Lendahl U (2011) Notch signaling: simplicity in design, versatility in function. Development 138(17):3593–3612. doi:10.1242/dev.063610
Iso T, Hamamori Y, Kedes L (2003) Notch signaling in vascular development. Arterioscler Thromb Vasc Biol 23(4):543–553. doi:10.1161/01.atv.0000060892.81529.8f
Liu J, Sato C, Cerletti M, Wagers A (2010) Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol 92:367–409. doi:10.1016/s0070-2153(10)92012-7
Bigas A, Robert-Moreno A, Espinosa L (2010) The Notch pathway in the developing hematopoietic system. Int J Dev Biol 54(6–7):1175–1188. doi:10.1387/ijdb.093049ab
Lewis J, Hanisch A, Holder M (2009) Notch signaling, the segmentation clock, and the patterning of vertebrate somites. J Biol 8(4):44. doi:10.1186/jbiol145
Williams R, Lendahl U, Lardelli M (1995) Complementary and combinatorial patterns of Notch gene family expression during early mouse development. Mech Dev 53(3):357–368
Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL (2004) Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 18(1):99–115. doi:10.1101/gad.276304
Loomes KM, Taichman DB, Glover CL, Williams PT, Markowitz JE, Piccoli DA, Baldwin HS, Oakey RJ (2002) Characterization of Notch receptor expression in the developing mammalian heart and liver. Am J Med Genet 112(2):181–189. doi:10.1002/ajmg.10592
Varadkar P, Kraman M, Despres D, Ma G, Lozier J, McCright B (2008) Notch2 is required for the proliferation of cardiac neural crest-derived smooth muscle cells. Dev Dyn 237(4):1144–1152. doi:10.1002/dvdy.21502
Noseda M, Chang L, McLean G, Grim JE, Clurman BE, Smith LL, Karsan A (2004) Notch activation induces endothelial cell cycle arrest and participates in contact inhibition: role of p21Cip1 repression. Mol Cell Biol 24(20):8813–8822. doi:10.1128/mcb.24.20.8813-8822.2004
Loomes KM, Underkoffler LA, Morabito J, Gottlieb S, Piccoli DA, Spinner NB, Baldwin HS, Oakey RJ (1999) The expression of Jagged1 in the developing mammalian heart correlates with cardiovascular disease in Alagille syndrome. Hum Mol Genet 8(13):2443–2449
van den Akker NM, Molin DG, Peters PP, Maas S, Wisse LJ, van Brempt R, van Munsteren CJ, Bartelings MM, Poelmann RE, Carmeliet P, de Groot ACG (2007) Tetralogy of Fallot and alterations in vascular endothelial growth factor-A signaling and notch signaling in mouse embryos solely expressing the VEGF120 isoform. Circ Res 100(6):842–849. doi:10.1161/01.res.0000261656.04773.39
Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J (2004) Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 18(20):2474–2478. doi:10.1101/gad.1239004
Benedito R, Duarte A (2005) Expression of Dll4 during mouse embryogenesis suggests multiple developmental roles. Gene Expr Patterns 5(6):750–755. doi:10.1016/j.modgep.2005.04.004
Leimeister C, Externbrink A, Klamt B, Gessler M (1999) Hey genes: a novel subfamily of hairy- and Enhancer of split related genes specifically expressed during mouse embryogenesis. Mech Dev 85(1–2):173–177
Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak TW, Honjo T (1995) Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development 121(10):3291–3301
Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T (2004) Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 18(20):2469–2473. doi:10.1101/gad.1239204
High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA (2008) Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci USA 105(6):1955–1959. doi:10.1073/pnas.0709663105
Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T (2000) Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 14(11):1343–1352
Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, Shou W, Ballestar E, Esteller M, Rojas A, Perez-Pomares JM, de la Pompa JL (2007) Notch signaling is essential for ventricular chamber development. Dev Cell 12(3):415–429. doi:10.1016/j.devcel.2006.12.011
McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T (2001) Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development 128(4):491–502
Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, Saijoh Y, O’Brien TP, Hamada H, Gridley T (2003) Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes Dev 17(10):1207–1212. doi:10.1101/gad.1084703
Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, Joutel A, Gridley T (2003) Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis 37(3):139–143. doi:10.1002/gene.10241
Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK (2005) Essential role of endothelial Notch1 in angiogenesis. Circulation 111(14):1826–1832. doi:10.1161/01.cir.0000160870.93058.dd
Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T (1999) Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet 8(5):723–730
Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, Weinmaster G, Gridley T (1998) Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev 12(7):1046–1057
Donovan J, Kordylewska A, Jan YN, Utset MF (2002) Tetralogy of Fallot and other congenital heart defects in Hey2 mutant mice. Curr Biol 12(18):1605–1610
Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M (2004) The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 18(8):901–911. doi:10.1101/gad.291004
Fischer A, Steidl C, Wagner TU, Lang E, Jakob PM, Friedl P, Knobeloch KP, Gessler M (2007) Combined loss of Hey1 and HeyL causes congenital heart defects because of impaired epithelial to mesenchymal transition. Circ Res 100(6):856–863. doi:10.1161/01.RES.0000260913.95642.3b
Acharya A, Hans CP, Koenig SN, Nichols HA, Galindo CL, Garner HR, Merrill WH, Hinton RB, Garg V (2011) Inhibitory role of Notch1 in calcific aortic valve disease. PLoS ONE 6(11):e27743. doi:10.1371/journal.pone.0027743
Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D (2005) Mutations in NOTCH1 cause aortic valve disease. Nature 437(7056):270–274. doi:10.1038/nature03940
Nigam V, Srivastava D (2009) Notch1 represses osteogenic pathways in aortic valve cells. J Mol Cell Cardiol 47(6):828–834. doi:10.1016/j.yjmcc.2009.08.008
Nus M, MacGrogan D, Martinez-Poveda B, Benito Y, Casanova JC, Fernandez-Aviles F, Bermejo J, de la Pompa JL (2011) Diet-induced aortic valve disease in mice haploinsufficient for the Notch pathway effector RBPJK/CSL. Arterioscler Thromb Vasc Biol 31(7):1580–1588. doi:10.1161/atvbaha.111.227561
Nakajima Y, Yamagishi T, Hokari S, Nakamura H (2000) Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat Rec 258(2):119–127
Shirai M, Imanaka-Yoshida K, Schneider MD, Schwartz RJ, Morisaki T (2009) T-box 2, a mediator of Bmp-Smad signaling, induced hyaluronan synthase 2 and Tgfbeta2 expression and endocardial cushion formation. Proc Natl Acad Sci USA 106(44):18604–18609. doi:10.1073/pnas.0900635106
Chang AC, Fu Y, Garside VC, Niessen K, Chang L, Fuller M, Setiadi A, Smrz J, Kyle A, Minchinton A, Marra M, Hoodless PA, Karsan A (2011) Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase. Dev Cell 21(2):288–300. doi:10.1016/j.devcel.2011.06.022
Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC (2008) Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem 283(12):7628–7637. doi:10.1074/jbc.M704883200
Townsend TA, Robinson JY, Deig CR, Hill CR, Misfeldt A, Blobe GC, Barnett JV (2011) BMP-2 and TGFbeta2 shared pathways regulate endocardial cell transformation. Cells Tissues Organs 194(1):1–12. doi:10.1159/000322035
Bharathy S, Xie W, Yingling JM, Reiss M (2008) Cancer-associated transforming growth factor beta type II receptor gene mutant causes activation of bone morphogenic protein-Smads and invasive phenotype. Cancer Res 68(6):1656–1666. doi:10.1158/0008-5472.can-07-5089
Daly AC, Randall RA, Hill CS (2008) Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol 28(22):6889–6902. doi:10.1128/mcb.01192-08
Candia AF, Watabe T, Hawley SH, Onichtchouk D, Zhang Y, Derynck R, Niehrs C, Cho KW (1997) Cellular interpretation of multiple TGF-beta signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development 124(22):4467–4480
Kluppel M, Wrana JL (2005) Turning it up a Notch: cross-talk between TGF beta and Notch signaling. BioEssays 27(2):115–118. doi:10.1002/bies.20187
Nieto MA (2002) The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 3(3):155–166. doi:10.1038/nrm757
Romano LA, Runyan RB (2000) Slug is an essential target of TGFbeta2 signaling in the developing chicken heart. Dev Biol 223(1):91–102. doi:10.1006/dbio.2000.9750
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2(2):76–83. doi:10.1038/35000025
Carver EA, Jiang R, Lan Y, Oram KF, Gridley T (2001) The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol 21(23):8184–8188. doi:10.1128/mcb.21.23.8184-8188.2001
Acknowledgments
AK and PAH are Senior Scholars of the Michael Smith Foundation for Health Research and are supported by the Heart and Stroke Foundation of Canada. VG is funded by a scholarship from the University of British Columbia. A special thanks to Rebecca Cullum for all her advice and help with editing this review.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Garside, V.C., Chang, A.C., Karsan, A. et al. Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development. Cell. Mol. Life Sci. 70, 2899–2917 (2013). https://doi.org/10.1007/s00018-012-1197-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1197-9