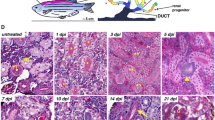
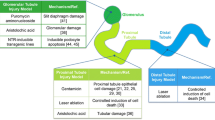
Abstract
The vertebrate kidney has an inherent ability to regenerate following acute damage. Successful regeneration of the injured kidney requires the rapid replacement of damaged tubular epithelial cells and reconstitution of normal tubular function. Identifying the cells that participate in the regeneration process as well as the molecular mechanisms involved may reveal therapeutic targets for the treatment of kidney disease. Renal regeneration is associated with the expression of genetic pathways that are necessary for kidney organogenesis, suggesting that the regenerating tubular epithelium may be “reprogrammed” to a less-differentiated, progenitor state. This review will highlight data from various vertebrate models supporting the hypothesis that nephrogenic genes are reactivated as part of the process of kidney regeneration following acute kidney injury (AKI). Emphasis will be placed on the reactivation of developmental pathways and how our understanding of the resulting regeneration process may be enhanced by lessons learned in the embryonic kidney.

Similar content being viewed by others
References
Hoffman PN, Cleveland DW (1988) Neurofilament and tubulin expression recapitulates the developmental program during axonal regeneration: induction of a specific beta-tubulin isotype. Proc Natl Acad Sci U S A 85:4530–4533
Tsonis PA (2000) Regeneration in vertebrates. Dev Biol 221:273–284
Tseng AS, Levin M (2008) Tail regeneration in Xenopus laevis as a model for understanding tissue repair. J Dent Res 87:806–816
Liu Y (2011) Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7:684–696
Murugan R, Kellum JA (2011) Acute kidney injury: what’s the prognosis? Nat Rev Nephrol 7:209–217
Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M (2008) Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol 294:C22–C28
Davidson AJ (2011) Uncharted waters: nephrogenesis and renal regeneration in fish and mammals. Pediatr Nephrol 26:1435–1443
Basile DP, Hammerman MR (1998) TGF-beta in renal development and renal growth. Miner Electrolyte Metab 24:144–148
Guo JK, Cantley LG (2010) Cellular maintenance and repair of the kidney. Annu Rev Physiol 72:357–376
Villanueva S, Cespedes C, Vio CP (2006) Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol 290:R861–R870
Al-Awqati Q, Oliver JA (2002) Stem cells in the kidney. Kidney Int 61:387–395
Saxen L, Sariola H (1987) Early organogenesis of the kidney. Pediatr Nephrol 1:385–392
James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM (2006) Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 133:2995–3004
James RG, Schultheiss TM (2005) Bmp signaling promotes intermediate mesoderm gene expression in a dose-dependent, cell-autonomous and translation-dependent manner. Dev Biol 288:113–125
Mauch TJ, Yang G, Wright M, Smith D, Schoenwolf GC (2000) Signals from trunk paraxial mesoderm induce pronephros formation in chick intermediate mesoderm. Dev Biol 220:62–75
Vize PD (2003) The chloride conductance channel ClC-K is a specific marker for the Xenopus pronephric distal tubule and duct. Gene Expr Patterns 3:347–350
Fox H (1963) The amphibian pronephros. Q Rev Biol 38:1–25
Marshman E, Booth C, Potten CS (2002) The intestinal epithelial stem cell. Bioessays 24:91–98
Goldstein J, Horsley V (2012) Home sweet home: skin stem cell niches. Cell Mol Life Sci 69:2573–2582
Lameire N, Van Biesen W, Vanholder R (2006) The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol 2:364–377
Kaushal GP, Basnakian AG, Shah SV (2004) Apoptotic pathways in ischemic acute renal failure. Kidney Int 66:500–506
Sheridan AM, Bonventre JV (2000) Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr Opin Nephrol Hypertens 9:427–434
Thadhani R, Pascual M, Bonventre JV (1996) Acute renal failure. N Engl J Med 334:1448–1460
Lash LH (2011) Renal membrane transport of glutathione in toxicology and disease. Vet Pathol 48:408–419
Goligorsky MS, Lieberthal W, Racusen L, Simon EE (1993) Integrin receptors in renal tubular epithelium: new insights into pathophysiology of acute renal failure. Am J Physiol 264:F1–F8
Molitoris BA, Marrs J (1999) The role of cell adhesion molecules in ischemic acute renal failure. Am J Med 106:583–592
Cianciolo Cosentino C, Roman BL, Drummond IA, Hukriede NA (2010) Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp 42:2079
Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, Chiba T, Novitskaya T, Woods C, West J, Korotchenko VN, McDermott L, Day BW, Davidson AJ, Harris RC, de Caestecker MP, Hukriede NA (2013) Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol 24:943–953
Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV (2005) Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol 288:F923–F929
Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, Zhu H, Ikenaga T, Ono F, Englert C, Cowan CA, Hukriede NA, Handin RI, Davidson AJ (2011) Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 470:95–100
Vogetseder A, Palan T, Bacic D, Kaissling B, Le Hir M (2007) Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol 292:C807–C813
Belleri M, Ribatti D, Nicoli S, Cotelli F, Forti L, Vannini V, Stivala LA, Presta M (2005) Antiangiogenic and vascular-targeting activity of the microtubule-destabilizing trans-resveratrol derivative 3,5,4′-trimethoxystilbene. Mol Pharmacol 67:1451–1459
Verghese E, Weidenfeld R, Bertram JF, Ricardo SD, Deane JA (2008) Renal cilia display length alterations following tubular injury and are present early in epithelial repair. Nephrol Dial Transplant 23:834–841
Witzgall R, Brown D, Schwarz C, Bonventre JV (1994) Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93:2175–2188
Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV (2008) Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2:284–291
Maeshima A, Yamashita S, Nojima Y (2003) Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol 14:3138–3146
Cantz T, Manns MP, Ott M (2008) Stem cells in liver regeneration and therapy. Cell Tissue Res 331:271–282
Stripp BR, Reynolds SD (2008) Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 5:328–333
Teta M, Rankin MM, Long SY, Stein GM, Kushner JA (2007) Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 12:817–826
Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P (2007) Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol 18:3128–3138
Hopkins C, Li J, Rae F, Little MH (2009) Stem cell options for kidney disease. J Pathol 217:265–281
Pleniceanu O, Harari-Steinberg O, Dekel B (2010) Concise review: kidney stem/progenitor cells: differentiate, sort out, or reprogram? Stem Cells 28:1649–1660
Sagrinati C, Ronconi E, Lazzeri E, Lasagni L, Romagnani P (2008) Stem-cell approaches for kidney repair: choosing the right cells. Trends Mol Med 14:277–285
Zubko R, Frishman W (2009) Stem cell therapy for the kidney? Am J Ther 16:247–256
Kobayashi T, Terada Y, Kuwana H, Tanaka H, Okado T, Kuwahara M, Tohda S, Sakano S, Sasaki S (2008) Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int 73:1240–1250
Lin F, Moran A, Igarashi P (2005) Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115:1756–1764
Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S (2003) Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol 14:1223–1233
Duester G (2008) Retinoic acid synthesis and signaling during early organogenesis. Cell 134:921–931
Ross SA, McCaffery PJ, Drager UC, De Luca LM (2000) Retinoids in embryonal development. Physiol Rev 80:1021–1054
Marill J, Idres N, Capron CC, Nguyen E, Chabot GG (2003) Retinoic acid metabolism and mechanism of action: a review. Curr Drug Metab 4:1–10
Chambon P (1994) The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol 5:115–125
Kastner P, Mark M, Ghyselinck N, Krezel W, Dupe V, Grondona JM, Chambon P (1997) Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development 124:313–326
Cartry J, Nichane M, Ribes V, Colas A, Riou JF, Pieler T, Dolle P, Bellefroid EJ, Umbhauer M (2006) Retinoic acid signalling is required for specification of pronephric cell fate. Dev Biol 299:35–51
Taira M, Jamrich M, Good PJ, Dawid IB (1992) The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev 6:356–366
Moriya N, Uchiyama H, Asashima M (1993) Induction of pronephric tubules by activin and retinoic acid in presumptive ectoderm of Xenopus laevis. Dev Growth Differ 35:123–128
Osafune K, Nishinakamura R, Komazaki S, Asashima M (2002) In vitro induction of the pronephric duct in Xenopus explants. Dev Growth Differ 44:161–167
Taira M, Otani H, Jamrich M, Dawid IB (1994) Expression of the LIM class homeobox gene Xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development 120:1525–1536
Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP, Davidson AJ (2007) The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet 3:1922–1938
Preger-Ben Noon E, Barak H, Guttmann-Raviv N, Reshef R (2009) Interplay between activin and Hox genes determines the formation of the kidney morphogenetic field. Development 136:1995–2004
Wingert RA, Davidson AJ (2011) Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev Dyn 240:2011–2027
Lelievre-Pegorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, Merlet-Benichou C (1998) Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int 54:1455–1462
Wilson JG, Roth CB, Warkany J (1953) An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat 92:189–217
Wilson JG, Warkany J (1948) Malformations in the genito-urinary tract induced by maternal vitamin A deficiency in the rat. Am J Anat 83:357–407
Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M (1994) Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 120:2749–2771
Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C (2001) Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet 27:74–78
Huang CC, Huang JK (2011) Sepsis-induced acute bilateral renal cortical necrosis. Nephrology (Carlton) 16:787
Takenaga M, Ohta Y, Tokura Y, Hamaguchi A, Shudo K, Okano H, Igarashi R (2009) The effect of Am-80, a synthetic retinoid, on spinal cord injury-induced motor dysfunction in rats. Biol Pharm Bull 32:225–231
Van Neerven S, Mey J, Joosten EA, Steinbusch HW, van Kleef M, Marcus MA, Deumens R (2010) Systemic but not local administration of retinoic acid reduces early transcript levels of pro-inflammatory cytokines after experimental spinal cord injury. Neurosci Lett 485:21–25
Wong LF, Yip PK, Battaglia A, Grist J, Corcoran J, Maden M, Azzouz M, Kingsman SM, Kingsman AJ, Mazarakis ND, McMahon SB (2006) Retinoic acid receptor beta2 promotes functional regeneration of sensory axons in the spinal cord. Nat Neurosci 9:243–250
Yip PK, Wong LF, Pattinson D, Battaglia A, Grist J, Bradbury EJ, Maden M, McMahon SB, Mazarakis ND (2006) Lentiviral vector expressing retinoic acid receptor beta2 promotes recovery of function after corticospinal tract injury in the adult rat spinal cord. Hum Mol Genet 15:3107–3118
Massaro GD, Massaro D (1997) Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med 3:675–677
Tepper J, Pfeiffer J, Aldrich M, Tumas D, Kern J, Hoffman E, McLennan G, Hyde D (2000) Can retinoic acid ameliorate the physiologic and morphologic effects of elastase instillation in the rat? Chest 117:242S–244S
Stinchcombe SV, Maden M (2008) Retinoic acid induced alveolar regeneration: critical differences in strain sensitivity. Am J Respir Cell Mol Biol 38:185–191
Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD (2011) Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell 20:397–404
Stuckmann I, Evans S, Lassar AB (2003) Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev Biol 255:334–349
Wagner J, Dechow C, Morath C, Lehrke I, Amann K, Waldherr R, Floege J, Ritz E (2000) Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J Am Soc Nephrol 11:1479–1487
Suzuki A, Ito T, Imai E, Yamato M, Iwatani H, Kawachi H, Hori M (2003) Retinoids regulate the repairing process of the podocytes in puromycin aminonucleoside-induced nephrotic rats. J Am Soc Nephrol 14:981–991
Perez A, Ramirez-Ramos M, Calleja C, Martin D, Namorado MC, Sierra G, Ramirez-Ramos ME, Paniagua R, Sanchez Y, Arreola L, Reyes JL (2004) Beneficial effect of retinoic acid on the outcome of experimental acute renal failure. Nephrol Dial Transplant 19:2464–2471
Frigerio G, Burri M, Bopp D, Baumgartner S, Noll M (1986) Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell 47:735–746
Asano M, Gruss P (1992) Pax-5 is expressed at the midbrain-hindbrain boundary during mouse development. Mech Dev 39:29–39
Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P (1990) Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 109:787–795
Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P (1990) Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 110:643–651
Mansouri A, Chowdhury K, Gruss P (1998) Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet 19:87–90
Torres M, Gomez-Pardo E, Dressler GR, Gruss P (1995) Pax-2 controls multiple steps of urogenital development. Development 121:4057–4065
Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M (2002) Nephric lineage specification by Pax2 and Pax8. Genes Dev 16:2958–2970
Majumdar A, Lun K, Brand M, Drummond IA (2000) Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development 127:2089–2098
Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arumae U, Meng X, Lindahl M, Pachnis V, Sariola H (1997) Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development 124:4077–4087
Vega QC, Worby CA, Lechner MS, Dixon JE, Dressler GR (1996) Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc Natl Acad Sci U S A 93:10657–10661
Brophy PD, Ostrom L, Lang KM, Dressler GR (2001) Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128:4747–4756
Gong KQ, Yallowitz AR, Sun H, Dressler GR, Wellik DM (2007) A Hox-Eya-Pax complex regulates early kidney developmental gene expression. Mol Cell Biol 27:7661–7668
Clarke JC, Patel SR, Raymond RM Jr, Andrew S, Robinson BG, Dressler GR, Brophy PD (2006) Regulation of c-Ret in the developing kidney is responsive to Pax2 gene dosage. Hum Mol Genet 15:3420–3428
Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M (2007) Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol 18:1121–1129
Imgrund M, Grone E, Grone HJ, Kretzler M, Holzman L, Schlondorff D, Rothenpieler UW (1999) Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney Int 56:1423–1431
Maeshima A, Maeshima K, Nojima Y, Kojima I (2002) Involvement of Pax-2 in the action of activin A on tubular cell regeneration. J Am Soc Nephrol 13:2850–2859
Zhang SL, Guo J, Moini B, Ingelfinger JR (2004) Angiotensin II stimulates Pax-2 in rat kidney proximal tubular cells: impact on proliferation and apoptosis. Kidney Int 66:2181–2192
Cohen T, Loutochin O, Amin M, Capolicchio JP, Goodyer P, Jednak R (2007) PAX2 is reactivated in urinary tract obstruction and partially protects collecting duct cells from programmed cell death. Am J Physiol Renal Physiol 292:F1267–F1273
Carroll TJ, Vize PD (1999) Synergism between Pax-8 and lim-1 in embryonic kidney development. Dev Biol 214:46–59
Cirio MC, Hui Z, Haldin CE, Cosentino CC, Stuckenholz C, Chen X, Hong SK, Dawid IB, Hukriede NA (2011) Lhx1 is required for specification of the renal progenitor cell field. PLoS One 6:e18858
Shawlot W, Behringer RR (1995) Requirement for Lim1 in head-organizer function. Nature 374:425–430
Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, Kania A, Jessell TM, Behringer RR, Tam PP (2000) Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev Biol 223:77–90
Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR (2005) Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132:2809–2823
Potter SS, Hartman HA, Kwan KM, Behringer RR, Patterson LT (2007) Laser capture-microarray analysis of Lim1 mutant kidney development. Genesis 45:432–439
Karavanov AA, Karavanova I, Perantoni A, Dawid IB (1998) Expression pattern of the rat Lim-1 homeobox gene suggests a dual role during kidney development. Int J Dev Biol 42:61–66
Kuhnlein RP, Frommer G, Friedrich M, Gonzalez-Gaitan M, Weber A, Wagner-Bernholz JF, Gehring WJ, Jackle H, Schuh R (1994) spalt encodes an evolutionarily conserved zinc finger protein of novel structure which provides homeotic gene function in the head and tail region of the Drosophila embryo. EMBO J 13:168–179
De Celis JF, Barrio R (2009) Regulation and function of Spalt proteins during animal development. Int J Dev Biol 53:1385–1398
Onuma Y, Nishinakamura R, Takahashi S, Yokota T, Asashima M (1999) Molecular cloning of a novel Xenopus spalt gene (Xsal-3). Biochem Biophys Res Commun 264:151–156
Hollemann T, Schuh R, Pieler T, Stick R (1996) Xenopus Xsal-1, a vertebrate homolog of the region specific homeotic gene spalt of Drosophila. Mech Dev 55:19–32
Farrell ER, Tosh G, Church E, Munsterberg AE (2001) Cloning and expression of CSAL2, a new member of the spalt gene family in chick. Mech Dev 102:227–230
Camp E, Hope R, Kortschak RD, Cox TC, Lardelli M (2003) Expression of three spalt (sal) gene homologues in zebrafish embryos. Dev Genes Evol 213:35–43
Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W (1998) Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet 18:81–83
Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, Katsuki M, Asashima M, Yokota T (2001) Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128:3105–3115
Kiefer SM, Ohlemiller KK, Yang J, McDill BW, Kohlhase J, Rauchman M (2003) Expression of a truncated Sall1 transcriptional repressor is responsible for Townes-Brocks syndrome birth defects. Hum Mol Genet 12:2221–2227
Kiefer SM, Robbins L, Stumpff KM, Lin C, Ma L, Rauchman M (2010) Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development. Development 137:3099–3106
Abedin MJ, Imai N, Rosenberg ME, Gupta S (2011) Identification and characterization of Sall1-expressing cells present in the adult mouse kidney. Nephron Exp Nephrol 119:e75–e82
Osafune K, Takasato M, Kispert A, Asashima M, Nishinakamura R (2006) Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development 133:151–161
Obara-Ishihara T, Kuhlman J, Niswander L, Herzlinger D (1999) The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development 126:1103–1108
Bracken CM, Mizeracka K, McLaughlin KA (2008) Patterning the embryonic kidney: BMP signaling mediates the differentiation of the pronephric tubules and duct in Xenopus laevis. Dev Dyn 237:132–144
Dudley AT, Robertson EJ (1997) Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn 208:349–362
Martinez G, Mishina Y, Bertram JF (2002) BMPs and BMP receptors in mouse metanephric development: in vivo and in vitro studies. Int J Dev Biol 46:525–533
Goncalves A, Zeller R (2011) Genetic analysis reveals an unexpected role of BMP7 in initiation of ureteric bud outgrowth in mouse embryos. PLoS One 6:e19370
Dudley AT, Godin RE, Robertson EJ (1999) Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13:1601–1613
Dudley AT, Lyons KM, Robertson EJ (1995) A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9:2795–2807
Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G (1995) BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9:2808–2820
Kazama I, Mahoney Z, Miner JH, Graf D, Economides AN, Kreidberg JA (2008) Podocyte-derived BMP7 is critical for nephron development. J Am Soc Nephrol 19:2181–2191
Zhang C, Evans T (1996) BMP-like signals are required after the midblastula transition for blood cell development. Dev Genet 18:267–278
Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL (1997) Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol 188:235–247
Winnier G, Blessing M, Labosky PA, Hogan BL (1995) Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9:2105–2116
Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I (2000) Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105:863–873
Cain JE, Bertram JF (2006) Ureteric branching morphogenesis in BMP4 heterozygous mutant mice. J Anat 209:745–755
Cain JE, Nion T, Jeulin D, Bertram JF (2005) Exogenous BMP-4 amplifies asymmetric ureteric branching in the developing mouse kidney in vitro. Kidney Int 67:420–431
Raatikainen-Ahokas A, Hytonen M, Tenhunen A, Sainio K, Sariola H (2000) BMP-4 affects the differentiation of metanephric mesenchyme and reveals an early anterior-posterior axis of the embryonic kidney. Dev Dyn 217:146–158
Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R (2007) Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134:2397–2405
Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A (2004) Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 131:3401–3410
Ueda H, Miyazaki Y, Matsusaka T, Utsunomiya Y, Kawamura T, Hosoya T, Ichikawa I (2008) Bmp in podocytes is essential for normal glomerular capillary formation. J Am Soc Nephrol 19:685–694
Yanagita M, Oka M, Watabe T, Iguchi H, Niida A, Takahashi S, Akiyama T, Miyazono K, Yanagisawa M, Sakurai T (2004) USAG-1: a bone morphogenetic protein antagonist abundantly expressed in the kidney. Biochem Biophys Res Commun 316:490–500
Kopp JB (2000) BMP receptors in kidney. Kidney Int 58:2237–2238
Wetzel P, Haag J, Campean V, Goldschmeding R, Atalla A, Amann K, Aigner T (2006) Bone morphogenetic protein-7 expression and activity in the human adult normal kidney is predominantly localized to the distal nephron. Kidney Int 70:717–723
Almanzar MM, Frazier KS, Dube PH, Piqueras AI, Jones WK, Charette MF, Paredes AL (1998) Osteogenic protein-1 mRNA expression is selectively modulated after acute ischemic renal injury. J Am Soc Nephrol 9:1456–1463
Simon M, Maresh JG, Harris SE, Hernandez JD, Arar M, Olson MS, Abboud HE (1999) Expression of bone morphogenetic protein-7 mRNA in normal and ischemic adult rat kidney. Am J Physiol 276:F382–F389
Gould SE, Day M, Jones SS, Dorai H (2002) BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int 61:51–60
Hruska KA, Guo G, Wozniak M, Martin D, Miller S, Liapis H, Loveday K, Klahr S, Sampath TK, Morrissey J (2000) Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol 279:F130–F143
Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H, Ryan S, Griffiths D, Maliakal J, Jelic M, Pastorcic M, Stavljenic A, Sampath TK (1998) Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest 102:202–214
Zeisberg M, Bottiglio C, Kumar N, Maeshima Y, Strutz F, Muller GA, Kalluri R (2003) Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol 285:F1060–F1067
Zeisberg M, Muller GA, Kalluri R (2004) Are there endogenous molecules that protect kidneys from injury? The case for bone morphogenic protein-7 (BMP-7). Nephrol Dial Transplant 19:759–761
Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R (2003) BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9:964–968
Zeisberg M, Shah AA, Kalluri R (2005) Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem 280:8094–8100
Miller RK, McCrea PD (2010) Wnt to build a tube: contributions of Wnt signaling to epithelial tubulogenesis. Dev Dyn 239:77–93
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26
Saulnier DM, Ghanbari H, Brandli AW (2002) Essential function of Wnt-4 for tubulogenesis in the Xenopus pronephric kidney. Dev Biol 248:13–28
Naylor RW, Jones EA (2009) Notch activates Wnt-4 signalling to control medio-lateral patterning of the pronephros. Development 136:3585–3595
Roel G, Gent YY, Peterson-Maduro J, Verbeek FJ, Destree O (2009) Lef1 plays a role in patterning the mesoderm and ectoderm in Xenopus tropicalis. Int J Dev Biol 53:81–89
Lyons JP, Miller RK, Zhou X, Weidinger G, Deroo T, Denayer T, Park JI, Ji H, Hong JY, Li A, Moon RT, Jones EA, Vleminckx K, Vize PD, McCrea PD (2009) Requirement of Wnt/beta-catenin signaling in pronephric kidney development. Mech Dev 126:142–159
Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP (2005) Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9:283–292
Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP (2003) Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130:3175–3185
Stark K, Vainio S, Vassileva G, McMahon AP (1994) Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372:679–683
Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP (2009) A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136:161–171
Bridgewater D, Cox B, Cain J, Lau A, Athaide V, Gill PS, Kuure S, Sainio K, Rosenblum ND (2008) Canonical WNT/beta-catenin signaling is required for ureteric branching. Dev Biol 317:83–94
Karner CM, Dietrich MF, Johnson EB, Kappesser N, Tennert C, Percin F, Wollnik B, Carroll TJ, Herz J (2010) Lrp4 regulates initiation of ureteric budding and is crucial for kidney formation–a mouse model for Cenani-Lenz syndrome. PLoS One 5:e10418
Kuure S, Popsueva A, Jakobson M, Sainio K, Sariola H (2007) Glycogen synthase kinase-3 inactivation and stabilization of beta-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. J Am Soc Nephrol 18:1130–1139
Nguyen HT, Thomson AA, Kogan BA, Baskin LS, Cunha GR (1999) Expression of the Wnt gene family during late nephrogenesis and complete ureteral obstruction. Lab Invest 79:647–658
Surendran K, McCaul SP, Simon TC (2002) A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol 282:F431–F441
Dirocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD (2013) Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol. doi:10.1681/ASN.2012050512
Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS (2010) Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107:4194–4199
Kudoh T, Tsang M, Hukriede NA, Chen X, Dedekian M, Clarke CJ, Kiang A, Schultz S, Epstein JA, Toyama R, Dawid IB (2001) A gene expression screen in zebrafish embryogenesis. Genome Res 11:1979–1987
Thisse B, Heyer V, Lux A, Alunni V, Degrave A, Seiliez I, Kirchner J, Parkhill JP, Thisse C (2004) Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol 77:505–519
Tsang M, Friesel R, Kudoh T, Dawid IB (2002) Identification of Sef, a novel modulator of FGF signalling. Nat Cell Biol 4:165–169
Rosselot C, Spraggon L, Chia I, Batourina E, Riccio P, Lu B, Niederreither K, Dolle P, Duester G, Chambon P, Costantini F, Gilbert T, Molotkov A, Mendelsohn C (2010) Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137:283–292
Schaier M, Jocks T, Grone HJ, Ritz E, Wagner J (2003) Retinoid agonist isotretinoin ameliorates obstructive renal injury. J Urol 170:1398–1402
Torban E, Eccles MR, Favor J, Goodyer PR (2000) PAX2 suppresses apoptosis in renal collecting duct cells. Am J Pathol 157:833–842
Carroll T, Wallingford J, Seufert D, Vize PD (1999) Molecular regulation of pronephric development. Curr Top Dev Biol 44:67–100
Pedersen A, Skjong C, Shawlot W (2005) Lim 1 is required for nephric duct extension and ureteric bud morphogenesis. Dev Biol 288:571–581
Tucker JA, Mintzer KA, Mullins MC (2008) The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell 14:108–119
Acknowledgments
Supported by grants from the National Institutes of Health, NAH: 2R01 DK069403, 2R01 HD053287, 1P30 DK079307; MPdC: 1RO1 HL093057, 1P30 DK079341; NAH/MPdC 1RC4 DK090770; AJD: The Rutherford Foundation Trust and the Marsden Fund of New Zealand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cirio, M.C., de Groh, E.D., de Caestecker, M.P. et al. Kidney regeneration: common themes from the embryo to the adult. Pediatr Nephrol 29, 553–564 (2014). https://doi.org/10.1007/s00467-013-2597-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2597-2