Abstract.
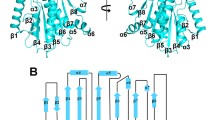
Histone acetyltranferase (HAT) enzymes are the catalytic subunit of large multisubunit HAT complexes that acetylate the ε-amino group of specific lysine residues on histone tails to promote transcriptional activation. Recent structural and functional studies on the divergent HAT enzymes Gcn5/PCAF, Esa1 and Hat1 have provided new insights into the underlying mechanism of histone binding and acetylation by HAT proteins. The three HAT enzymes contain a structurally conserved core domain that plays a functionally conserved role in binding the coenzyme A cofactor and in harboring the putative general base for catalysis. Structurally variable N- and C-terminal domains appear to contain a related scaffold that mediates histone substrate binding. These data provide a framework for understanding the structure and function of other more divergent HAT proteins such as TAFII250 and CBP/p300, and provides a starting point for understanding how HAT proteins may cooperate with other factors within in vivo HAT complexes to promote transcriptional activation.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Rights and permissions
About this article
Cite this article
Marmorstein, R. Structure and function of histone acetyltransferases. CMLS, Cell. Mol. Life Sci. 58, 693–703 (2001). https://doi.org/10.1007/PL00000893
Issue Date:
DOI: https://doi.org/10.1007/PL00000893