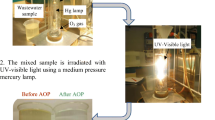
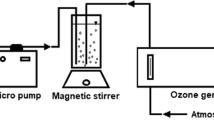
Abstract
It is known that hydrogen peroxide interferes with chemical oxygen demand analysis by consuming oxidation agents such as potassium dichromate, thus leading to overestimation of the chemical oxygen demand measurements. The objective of the study was to investigate the effects of hydrogen peroxide interference and to determine true chemical oxygen demand values on interpreting treatment performance during ozone-based advanced oxidation of livestock wastewater in which hydrogen peroxide concentration and chemical oxygen demand values are dynamically changing. According to the chemical oxygen demand monitoring data, chemical oxygen demand values were always higher than the initial chemical oxygen demand load when hydrogen peroxide was involved and the treatment performance with ozone alone or ozone/ultraviolet was better than with coupled hydrogen peroxide. The extent of overestimation was proportional to the remaining hydrogen peroxide concentration and the average overestimation ratio in livestock wastewater was in the range of 0.50∼0.58 mg per 1 mg of hydrogen peroxide, depending upon the quality of the wastewater treated. True chemical oxygen demand values were estimated by correlating the extent of overestimation with the remaining hydrogen peroxide concentration during treatment. The extent of overestimation decreased to zero gradually as the amount of hydrogen peroxide also approached zero as oxidation proceeded. The corrected chemical oxygen demand values indicated underlying tendency of oxidation, which could not be seen in the original chemical oxygen demand monitoring data. Application of ozone/hydrogen peroxide was more efficient for reducing chemical oxygen demand than ozone alone, as was ozone/hydrogen peroxide/ultraviolet compared to ozone/ultraviolet. When coupled with ozone, ultraviolet irradiation was more efficient than hydrogen peroxide for decreasing chemical oxygen demand during treatment of livestock wastewater.
Similar content being viewed by others
References
Adams, R. H.; Ola¡n-Castro, D.; Guzma¡n-Osorio, F. J.; Daaz-Ramirez, I. J., (2009). Relationship between geomorphology and contamination with weathered hydrocarbons in an old river levee/ marsh association. Int. J. Environ. Sci. Tech., 6(4), 527–538 (12 Pages)
APHA; AWWA; WEF, (2005). Standard methods for the examination of water and wastewater. 21st Ed., American Public Health Association, American Water Works Association and the Water Environment Federation. Washington DC., USA.
Arslan, I.; Balcioglu, I., (2001). Advanced oxidation of raw and biotreated textile industry wastewater with O3, H2O2/UV-C and their sequential application. J. Chem. Tech. Biotech., 76(1), 53–60 (8 pages).
Bader, H.; Hoigne, J., (1981). Determination of ozone in water by the indigo method. Water Res., 15(4), 449–456 (8 pages).
Bandyopadhyay, G.; Chattopadhyay, S., (2007). Single hidden layer artificial neural network models versus multiple linear regression model in forecasting the time series of total ozone. Int. J. Environ. Sci. Tech., 4(1), 141–150 (10 Pages)
Barker, R.; Jones, A. R., (1988). Treatment of maloderants in air by UV/ozone technique. Ozone Sci. Eng., 10(4), 405–417 (13 pages).
Baumann, F. J., (1974). Dichromate reflux chemical oxygen demand: A proposed method for chloride in highly saline wastes. Anal. Chem., 46(9), 1336–1338 (3 pages).
Domini, C. E.; Hidalgo, M.; Marken, F.; Canals, A., (2006). Comparison of three optimized digestion methods for rapid determination of chemical oxygen demand: Closed microwaves, open microwaves and ultrasound irradiation. Anal. Chim. Acta, 561(1–2), 210–217 (8 pages).
El Diwani, G.; El Rafie; S.; Hawash, S., (2009). Degradation of 2, 4, 6-trinitotoluene in aqueous solution by ozonation and multi-stage ozonation biological treatment. Int. J. Environ. Sci. Tech., 6(4), 619–628 (10 Pages).
Fronk, C. A., (1987). Destruction of volatile organic contaminants in drinking water by ozone treatment. Ozone Sci. Eng., 9(3), 265–288 (24 pages).
Gharbani, P.; Tabatabaii, S. M.; Mehrizad, A., (2008). Removal of Congo Red from textile wastewater by ozonation. Int. J. Environ. Sci. Tech., 5(4), 495–500 (6 pages).
Gunten, U., (2003). Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res., 37(7), 1443–1467 (15 pages).
HACH, (2009). Oxygen Demand, Chemical Method 8000. DR/ 4000 Procedure.
Hoigne, J.; Bader, H., (1983). Rate constants of reactions of ozone with organic and inorganic compounds in water. Water Res., 17(2), 185–194 (10 pages).
Juang, D. F.; Lee, C. H.; Hsueh, S. C., (2009). Chlorinated volatile organic compounds found near the water surface of heavily polluted rivers. Int. J. Environ. Sci. Tech., 6(4), 545–556 (12 Pages).
Kang, Y. W.; Cho, M. J.; Hwang, K. Y., (1999). Correction of hydrogen peroxide interference on standard chemical oxygen demand test. Water Res., 33(5), 1247–1251 (5 pages).
Kleiser, G.; Frimmel, F. H., (2000). Removal of precursors for disinfection by-products: differences between ozone- and OH-radical-induced oxidation. Sci. Total Environ., 256(1), 1–9 (9 pages).
Kosaka, K.; Yamada, H.; Matsui, S.; Echigo, S.; Shishida, K., (1998). Comparison among the methods for hydrogen peroxide measurements to evaluate advanced oxidation processes: Application of a spectrophotometric method using copper (II) ion and 2, 9-dimethyl-1, 10-phenanthroline. Environ. Sci. Tech., 32(23), 3821–3824 (4 pages).
Kuo, W. G., (1992). Decolorizing dye wastewater with Fenton’s reagent. Water Res., 26(7), 881–886 (6 pages).
Kwon, B. G.; Ryu, S.; Yoon, J., (2009). Determination of hydroxyl radical rate constans in a continuous flow system using competition kinetics. J. Ind. Eng. Chem., 15(6), 809–812 (4 pages).
Langlais, B.; Reckhow, D. A.; Brink, D. R., (1991). Ozone in water treatment: Application and engineering, Lewis Publishers.
Legrini, O.; Oliveros, E.; Braun, A. M., (1993). Photochemical processes for water treatment. Chem. Rev., 93(2), 671–698 (8 pages).
Oeller, H. J.; Demel, I.; Weinberger, G., (1997). Reduction in residual COD in biologically treated paper mill effluents by means of combined ozone and ozone/UV reactor stages. Water Sci. Tech., 35(2–3), 269–276 (8 pages).
Oneby, M. A.; Bromley, C. O.; Borchardt, J. H.; Harrison, D. S., (2010). Ozone treatment of secondary effluent at U. S. municipal wastewater treatment plants. Ozone Sci. Eng., 32(1), 43–55 (13 pages).
Rakness, K. L., (2005). Ozone in drinking water treatment: process design, operation, and optimization, AWWA.
Richardson, S. D.; Thruston, A. D.; Caughran, T. V.; Chen, P. H.; Collette, T. W.; Floyd, T. L., (1999). Identification of new ozone disinfection byproducts in drinking water. Environ. Sci. Tech., 33(19), 3368–3377 (10 pages).
Rosenfeldt, E. J.; Linden, K. G.; Canonica, S.; von Gunten, V., (2006). Comparison of the efficiency of OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Res., 40(20), 3695–3704 (10 pages).
Samarghandi, M. R.; Nouri, J.; Mesdaghinia, A. R.; Mahvi, A. H.; Nasseri, S.; Vaezi, F., (2007). Efficiency removal of phenol, lead and cadmium by means of UV/ TiO2/ H2O2 rocesses. Int. J. Environ. Sci. Tech., 4(1), 19–26 (8 Pages).
Sauze, N.; Laplanche, A.; Martin, G.; Paillard, H., (1991). A process of washing and ozonation to deodorize an atmosphere contaminated by sulfides. Ozone Sci. Eng., 13(3), 331–347 (18 pages).
Talinli, I.; Anderson, G. K., (1992). Interference of hydrogen peroxide on the standard COD test. Water Res., 26(1), 107–110 (4 pages).
Tambosi, J. L.; de Sena, R. F.; Gebhardt, W.; Moreira, R.; Jose, H. J.; Schroder, H. F., (2009). Physicochemical and advanced oxidation process: A comparison of elimination results of antibiotic compounds following an MBR treatment. Ozone Sci. Eng., 31(6), 428–435 (8 pages).
Tizaoui, C.; Bouselmi, L.; Mansouri, L.; Ghrabi, A., (2007). Landfill leachate treatment with ozone/hydrogen peroxide systems. J. Hazard. Mater., 140(1–2), 316–324 (9 pages).
Tosik, R.; Wiktorowski, S., (2001). Color removal and improvement of biodegradability of wastewater from dye production using ozone and hydrogen peroxide. Ozone Sci. Eng., 23(4), 295–302 (8 pages).
Vaidya, B.; Watson, S. W.; Coldiron, S. J.; Porter, M. D., (1997). Reduction of chloride ion interference in chemical oxygen demand determination using bismuth-based adsorbents. Anal. Chim. Acta, 357(1–2), 167–175 (8 pages).
Wu, J. J.; Muruganandham, M.; Chen, S. H., (2007). Degradation of DMSO by ozone-based advanced oxidation processes. J. Hazard. Mater., 149(1), 218–225 (8 pages).
Yasar, A.; Ahmad, N.; Chaudry, M. N.; Rehman, M. S. U.; Khan, A. A., (2007). Ozone for color and COD removal of raw and anaerobic biotreated combined industrial wastewater. Pol. J. Environ. Stud., 16(2), 289–294 (6 pages).
Zak, S., (2008). Problem of correction of the chemical oxygen demand values determined in wastewaters treated by methods with hydrogen peroxide. Proc. ECOpole, 2(2), 409–414 (5 pages).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, E., Lee, H., Kim, Y.K. et al. Hydrogen peroxide interference in chemical oxygen demand during ozone based advanced oxidation of anaerobically digested livestock wastewater. Int. J. Environ. Sci. Technol. 8, 381–388 (2011). https://doi.org/10.1007/BF03326225
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03326225