Abstract
Background: Prompt and accurate diagnosis of infectious endophthalmitis is crucial for rapid and effective treatment. By identifying whether the causative pathogen is bacterial or fungal, a rational approach for the use of antibacterials or corticosteroids, respectively, can be followed.
Aim: To assess the clinical utility of broad-range bacterial and fungal DNA amplification in the detection of endophthalmitis (postoperative, posttraumatic, and endogenous).
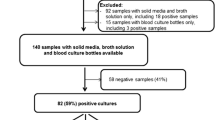
Methods: In a prospective study, vitreous humor samples from 70 patients with the clinical diagnosis of presumed endophthalmitis, and from 30 patients undergoing surgery for non-infectious causes, were subjected to routine microbiologic and molecular investigation. DNA extracted from a 50μL sample was amplified by primers targeting the conserved 16S and 18S ribosomal RNA gene sequences of bacteria and fungi, respectively. Reagents for bacterial DNA amplification were decontaminated of endogenous DNA using 8-methoxypsoralen and long wave UV treatment.
Results and Discussion: A total of 35 specimens were positive for bacteria or fungi by culture. Of these, Gram-positive organisms were isolated in 19 specimens, Gram-negative organisms in 13 specimens and fungi in 3 specimens. Pseudomonas species, coagulase-negative Staphylococcus, and Streptococcus species were the main etiological agents isolated. Bacterial DNA amplification resulted in 49 positive specimens, compared with 32 positive specimens by culture; and fungal DNA amplification resulted in 11 positive specimens, compared with 3 positive specimens by culture. All control specimens were negative for both culture and DNA amplification.
Conclusion: DNA extracted using a single-extraction protocol from 50μL of vitreous humor and amplified with broad-range bacterial and fungal primers will enable the rapid differentiation (within 14 hours) between bacterial and fungal endophthalmitis and allow tailoring of therapy to individual patients.







Similar content being viewed by others
References
Okhravi N, Adamson P, Matheson M, et al. PCR-based evidence of bacterial involvement in eyes with suspected intraocular infection. Invest Ophthalmol Vis Sci 2000; 41(11): 3474–9
Jaeger E, Carroll N, Choudhury S, et al. Rapid detection and identification of Candida, Aspergillus and Fusarium species in ocular samples using nested PCR. J Clin Microbiol 2000; 38(8): 2902–8
Harris K, Hartley JC. Development of broad-range 16S rDNA PCR for use in the routine diagnostic clinical microbiology service. J Med Microbiol 2003; 52: 685–91
Anand A, Madhavan HN, Neelam V. Use of polymerase chain reaction in the diagnosis of fungal endophthalmitis. Ophthalmology 2001; 108: 326–30
Hughes MS, Beck LA, Skuce RA. Identification and elimination of DNA sequences in Taq DNA polymerase. J Clin Microbiol 1994; 32(8): 2007–8
Meier A, Persing DH, Marion F, et al. Elimination of contaminating DNA within polymerase chain reaction reagents: implications for a general approach to detection of uncultured pathogens. J Clin Microbiol 1993; 31: 646–52
Corless CE, Guiver M, Borrow R, et al. Contamination and sensitivity issues with a real-time universal 16S rRNA PCR. J Clin Microbiol 2000; 38(5): 1747–52
Klaschik S, Lehmann L, Raadts A, et al. Comparison of different decontamination methods for reagents to detect low concentrations of bacterial 16S rDNA by real-time PCR. Mol Biotechnol 2002; 22: 231–42
Okhravi N, Adamson P, Lightman S. Polymerase chain reaction in the diagnosis of bacterial endophthalmitis. Br J Ophthalmol 1999; 83: 378–80
Megevand GS, Pournaras CJ. Current approach to postoperative endophthalmitis. Br J Ophthalmol 1997; 81: 1006–15
Han D, Wisniewski S, Wilson L, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol 1996; 122: 1–17
Millar C, Xu J, John E, et al. Risk assessment models and contamination management: implications for broad-range ribosomal DNA PCR as a diagnostic tool in medical bacteriology. J Clin Microbiol 2002; 40: 1575–80
Lohmann CP, Linde H-J, Reischl U. Improved detection of microorganisms by polymerase chain reaction in delayed endophthalmitis after cataract surgery. Ophthalmology 2000; 107: 1047–52
Therese L, Anand A, Madhavan HN. Polymerase chain reaction in the diagnosis of bacterial endophthalmitis. Br J Ophthalmol 1998; 82: 1078–82
Rowther F, Rodrigues C, Mehta A, et al. An improved method of elimination of DNA from PCR reagents. Mol Diagn 2005; 9(2): 53–7
Kunimoto D, Das T, Sharma S, et al. Microbiologic spectrum and susceptibility of isolates, part I: postoperative endophthalmitis. Am J Ophthalmol 1999; 128: 240–2
Anand A, Therese L, Madhavan HN. Spectrum of aetiological agents of postoperative endophthalmitis and antibiotic susceptibility of bacterial isolates. Indian J Ophthalmol 2000; 48: 123–8
Kunimoto D, Das T, Sharma S, et al. Microbiologic spectrum and susceptibility of isolates, part II: posttraumatic endophthalmitis. Am J Ophthalmol 1999; 128: 242–4
Therese L, Anand A, Madhavan HN. Spectrum of bacterial and fungal agents isolated from patients with endogenous endophthalmitis. Indian J Med Microbiol 1997; 15: 187–90
Sharma S, Das D, Anand R, et al. Reliability of nested polymerase chain reaction in the diagnosis of bacterial endophthalmitis. Am J Ophthalmol 2002; 133: 142–4
Carroll NM, Jaeger EEM, Choudhury S. Detection of and discrimination between Gram-positive and Gram-negative bacteria in intraocular samples by using nested PCR. J Clin Microbiol 2000 May; 38(5): 1753–7
Maiwald M. Broad-range PCR for detection and identification of bacteria. In: Persing DH, Tenover FC, Versalovic J, et al., editors. Molecular microbiology: diagnostic principles and practice. Washington, DC: ASM Press, 2004: 379–390
Mangiapan G, Vokurka M, Schouls L. Sequence capture-PCR improves detection of mycobacterial DNA in clinical specimens. J Clin Microbiol 1996; 34: 1209–15
Song J, Cho H, Park MY, et al. Detection of Salmonella typhi in the blood of patients with typhoid fever by polymerase chain reaction. J Clin Microbiol 1993; 31: 1439–43
Wilson I. Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol 1997; 63: 3741–51
Klotz S. Penn C, Negvesky G, et al. Fungal and parasitic infections of the eye. Clin Microbiol Rev 2000; 13: 662–85
Tang W, Persing DH. Molecular detection and identification of microorganisms. In: Murray PR, Baron EJ, Michael P, et al., editors. Manual of Clinical Microbiology. 7th ed. Washington, DC: ASM Press, 1999: 215–244
Jalava KR, Nikkari S, Jalava J. Direct amplification of rRNA genes in diagnosis of bacterial infections. J Clin Microbiol 2000; 38: 32–9
Acknowledgments
This research was funded by the National Health & Education Society, P.D. Hinduja National Hospital & Medical Research Center, Mumbai, India.
The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varghese, B., Rodrigues, C., Deshmukh, M. et al. Broad-Range Bacterial and Fungal DNA Amplification on Vitreous Humor from Suspected Endophthalmitis Patients. Mol Diag Ther 10, 319–326 (2006). https://doi.org/10.1007/BF03256207
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256207