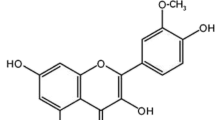
Summary
To develop a new HPLC-UV method of determining silybin in human plasma and to study the pharmacokinetic of silybin-phosphatidylcholine complex (silybinin capsules) in healthy male Chinese volunteers using the new developed method. The assays were validated over the concentration range of 3.5–14336.0 ng-ml−1 in human plasma. In either matrix, the lower limit of quantitation was 3.5 ng-ml−1. The intra- and inter-day precision were less than 10% in terms of RSD. The absolute recoverys were more than 90%. The validated assay was suitable for pharmacokinetic studies of silybin. In order to assess its pharmacokinetic profile in human, plasma silybin levels were determined after administration of single oral doses of silybin-phosphatidylcholine complex (equivalent to 280mg silybin) to 20 subjects. Silybin was absorpted rapidly, the times to reach peak plasma concentration (Tmax) ranged from 0.67 to 2.67h, and the mean was 1.4h. Other Pharmacokinetic parameters of silybin in human were Cmax 4242.1±2252.9 ng-ml−1; AUC(0–∞) 5946.6±1898.9 ng-h-ml−1; KeI 0.31±O.O8 h−1; t1/22.38±0.76 h; Ka5.48±2.00 h−1; CL55.0+28.1 L-h−1; Vd 191.7±125.1 L, respectively.
Similar content being viewed by others
References
Flora K, Hahn M, Rosen H, Benner K (1998): Milk thistle (Silybum marianum) for the therapy of liver diseases. Am J Gastroenterol, 93, 139–143.
Valenzuela A, Garrido A (1994): Biochemical bases of the pharmacological action of the flavonoid Silymarin and its structural isomersilybinin. Biol Res, 27, 105–112.
Morazzoni P, Bombardelli E (1995): Silybum marianum (Carduus marianus). Fitoterapia, 66, 3–42.
Skottova N, Krecman V (1998): Dietary Silymarin improves removal of low density lipoproteins by the perfused rat liver. Acta Univ Palacki Olomuc (Olomouc) Fac Med, 141, 39–40.
Skottova N, Krecman V, Simanek V (1999): Activities of Silymarin and its flavonolignans upon low density lipoprotein oxidizability in vitro. Phytother Res, 13, 535–537.
Barzaghi N, Crema F, Gatti G, Perucca E (1990): Pharmacokinetic studies on IdB 1016,asilybin-phosphatidylcholine complex, in healthy human subjects. Euro. J. Drug. Metab. Pharmacokinet., 15, 333–338.
Morazzoni P, Magistretti MJ, Giachetti C, Zanolo G (1992): Comparative bioavailability of silipide, a new flavanolignan complex, in rats. Euro. J. Drug. Metab. Pharmacokinet., 17, 39–44.
Morazzoni P, Montalbetti A, Malandrino S, Pifferi G (1993): Comparative pharmacokinetics of silipide and Silymarin in rats. Euro. J. Drug. Metab. Pharmacokinet., 18, 289–297.
Marisa C, Salvatore M, Maria JM (1992): Protective activity of silipide on liver damage in rodents. Japan J. Pharmacol., 60, 315–321.
Nina S, Zdenek S, Rostislav V, Karel U, Alexander J, Vilim S (2001): Pharmacokinetic study of iodine-labeled silybinins in rat. Pharmacological Research, 44, 247–253.
Gibaldi M, Perrier D (1982): Pharmacokinetics, 2nd edn. New York: Marcel Dekker, 409–417.
Cavallini L, Lucchetti G (1976): Cinsiderazioni clinicofarmacologiche sullasilimarina. Gazz. Med. It, 135, 365–374.
Schandalik R, Gatti G, Perucca EP (1992): harmacokinetics of silybin in bile following administration of silipide and Silymarin in cholecystectomy patients. Arzneimittelforschung, 42, 964–968.
Schandalik R, Gatti G, Perucca E (1994): Pharmacokinetics of silybin following oral administration of silipide in patients with extrahepatic biliary obstruction. Drugs Exp Clin Res, 20, 37–42.
Lorenz D, Lucker PW, Mennicke WH, Wetzelaberger N (1984): Pharmacokinetic studies with Silymarin in human serum and bile. Meth. Find. Exp. Clin. Pharmacol., 6, 655–661.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, W., Gao, J., Zhao, HZ. et al. Development of a HPLC-UV assay for silybin-phosphatidylcholine complex (silybinin capsules) and its pharmacokinetic study in healthy male Chinese volunteers. European Journal of Drug Metabolism and Pharmacokinetics 31, 265–270 (2006). https://doi.org/10.1007/BF03190466
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190466