Abstract
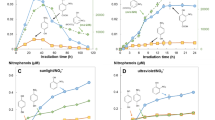
By means of simulated sunlight, the influence of natural organic matter (NOM) on the photochemical degradation of halogenated pesticides in the absence and presence of nitrate as a precursor of the highly reactive OH radicals in aqueous solutions and freshwater was investigated. Solutions of dichlorprop or terbutylazine (a) in phosphate-buffered demineralized water containing nitrate and/or NOM and (b) in natural freshwaters were irradiated by a 1000-W Xe short-arc lamp. The collimated beam was filtered using a combination of optical filters (WG 320 and WG 295) to simulate the solar spectrum under summer midday conditions. In the absence of nitrate and NOM, the pesticides were degraded photolytically by simulated sunlight. The degradation rates depended on the absorption spectrum in the UVB range and the quantum yield of the degradation. The photochemical degradation of the pesticides was faster in the presence of nitrate due to the sunlight-induced formation of OH radicals. In the absence of nitrate, low concentrations of NOM of a brownwater lake accelerated the degradation due to the formation of reactive species by NOM. At higher concentrations of NOM, the inner filter effect of NOM lowered the degradation rates. In the presence of 50 mg l−1 nitrate, NOM decreased the degradation rate significantly. In case the natural water samples were used as a matrix for the experiments (nitrate concentrations between 2 mg l−1 and 15 mg l−1, DOC concentrations below 2.3 mg l−1), NOM acted mainly as a radiation filter and as a scavenger of OH radicals. As a consequence, in most freshwater systems, the accelerating effect of NOM by the formation of reactive species is of minor importance compared to the inner filter effect and to radical scavenging.
Similar content being viewed by others
References
Braun, A. M.;Maurette, M.-T.;Oliveros, E. (1991): Photochemical Technology. John Wiley & Sons, Chichester
Brezonik, P.L.;Fulkerson-Brekken, J. (1998): Nitrate-Induced Photolysis in Natural Waters: Controls on Concentrations of Hydroxyl Radical Photo-Intermediates by Natural Scavenging Agents. Environ. Sci. Technol.32, 3004–3010
Buxton, G.V.;Greenstock, C.L.;Helman, W.P.;Ross, A.B. (1988): Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (·OH/·O−) in Aqueous Solution. J. Phys. Chem. Ref. Data17, 513–886
Chen, Y.;Khan, S.U.;Schnitzer, M. (1978): Ultraviolet Irradiation of Dilute Fulvic Acid Solutions. Soil Sci. Soc. Am. J.42, 292–296
Cooper, W.J.;Zika, R.G.;Petasne, R.G.;Fischer, A.M. (1989): Sunlight-Induced Photochemistry of Humic Substances in Natural Waters: Major Reactive Species. In Aquatic Humic Substances. Influence on Fate and Treatment of Pollutants (Edited bySuffet I. H. andMacCarthy P.), pp. 333–362. American Chemical Society, Washington, DC
Daniels, M.;Meyers, R.V.;Belardo, E. V. (1968): Photochemistry of the Aqueous Nitrate System. I. Excitation in the 300-mμ Band. J. Phys. Chem.72, 389–399
Frimmel, F.H. (1994): Photochemical Aspects Related to Humic Substances. Environ. Intern.20, 373–385
Frimmel, F.H.;Bauer, H.;Putzien, J.;Murasecco, P.;Braun, A.M. (1987): Laser Flash Photolysis of Dissolved Aquatic Humic Material and the Sensitized Production of Singlet Oxygen. Environ. Sci. Technol.21, 541–545
Haag, W.R.;Hoigné, J. (1985): Photo-Sensitized Oxidation in Natural Water via OH Radicals. Chemosphere14, 1659–1671
Hoigné, J.;Faust, B.;Haag, W.R.;Scully, F.E.;Zepp, R.G. (1989): Aquatic Humic Substances as Sources and Sinks of Photochemically Produced Transient Reactants. In Aquatic Humic Substances. Influence on Fate and Treatment of Pollutants (Edited bySuffet, I. H. andMacCarthy, P.), pp. 333–362. American Chemical Society, Washington, DC
Legrini, O.;Oliveros, E.;Braun, A.M.(1993): Photochemical Processes for Water Treatment. Chemical Reviews93, 671–698
Li, J.;Yu, Z.;Gao, M.;Zhang, L.;Cai, X.;Chao, F. (1996): Effect of Ultraviolet Irradiation on the Characteristics and Trihalomethanes Formation Potential of Humic Acid. Wat. Res.30, 347–350
Mark, G.;Korth, H.-G.;Schuchmann, H.-P.; von Sonntag, C. (1996): The Photochemistry of Aqueous Nitrate Ion Revisited. J. Photochem. Photobiol.A 101, 89–103
Miller, W.L. (1998): Effects of UV Radiation on Aquatic Humus: Principles and Experimental Considerations. In Aquatic Humic Substances: Ecology and Biogeochemistry (Edited byHessen, D.O.;Tranvik, L.J.), Springer-Verlag, Berlin
Schindelin, A.J.;Shahin, N.;Frimmel, F.H. (1997): Model Experiments Relating with the Photochemical Degradation of Terbuthylazin and Dichlorprop by Simulated Sunlight in the Presence of Nitrate (Modellversuche zum solaren photochemischen Abbau von Terbuthylazin und Dichlorprop mit Hilfe von simuliertem Sonnenlicht und in Gegenwart von Nitrat). Vom Wasser88, 383–392
Seckmeyer, G.;McKenzie, R.L. (1992): Increased Ultraviolet Radiation in New Zealand (45°S) Relative to Germany (48°N). Nature359, 135–137
Vaughan, P.P.;Blough, N.V. (1998): Photochemical Formation of Hydroxyl Radical by Constituents of Natural Waters. Environ. Sci. Technol.32, 2947–2953
Warneck, P.;Wurzinger, C. (1988): Product Quantum Yields for the 305-m Photodecomposition of NO3− in Aqueous Solution. J. Phys. Chem.92, 6278–6283
Zepp, R.G.;Callaghan, T.V.;Erickson, D.J. (1995): Effects of Increased Solar Ultraviolet Radiation on Biogeochemical Cycles. Ambio24, 181–187
Zepp, R.G.;Hoigné, J.;Bader, H. (1987): Nitrate-Induced Photooxidation of Trace Organic Chemicals in Water. Environ. Sci. Technol.21, 443–450
Zika, R.G.;Cooper, W.J. (1987): Photochemistry of Environmental Aquatic Systems. ACS Symposium Series 327, American Chemical Society, Washington, DC
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schindelin, A.J., Frimmel, F.H. Nitrate and natural organic matter in aqueous solutions irradiated by simulated sunlight. Environ. Sci. & Pollut. Res. 7, 205–210 (2000). https://doi.org/10.1007/BF02987349
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02987349