Abstract
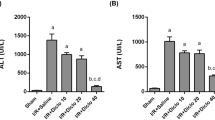
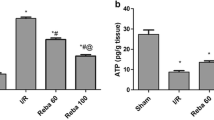
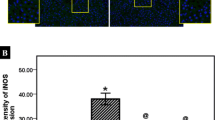
Reperfusion of ischemic liver results in the generation of oxygen radicals, nitric oxide (NO) and their reaction product peroxynitrite, all of which may cause strand breaks in DNA, which activate the nuclear enzyme poly(ADP ribose)synthase (PARS). This results in rapid depletion of intracellular nicotinamide adenine dinucleotide and adenosine 5′-triphosphate (ATP) and eventually induces irreversible cytotoxicity. In this study, we demonstrated that niacinamide, a PARS inhibitor, attenuated ischemia/reperfusion (I/R)-induced liver injury. Ischemia was induced by clamping the common hepatic artery and portal vein of rats for 40 min. Thereafter, flow was restored and the liver was reperfused for 90 min. Blood samples collected prior to I and after R were analyzed for methyl guanidine (MG), NO, tumor necrosis factor (TNF-α) and ATP. Blood levels of aspartate transferase (AST), alanine transferase (ALT) and lactate dehydrogenase (LDH) which served as indexes of liver injury were measured. This protocol resulted in elevation of the blood NO level (p<0.01). Inflammation was apparent, as TNF-α and MG levels were significantly increased (p<0.05 and p<0.001). AST, ALT and LDH were elevated 4-to 5-fold (p<0.001), while ATP was significantly diminished (p<0.01). After administration of niacinamide (10 mM), liver injury was significantly attenuated, while blood ATP content was reversed. In addition, MG, TNF-α and NO release was attenuated. These results indicate that niacinamide, presumably by acting with multiple functions, exerts potent anti-inflammatory effects in I/R-induced liver injury.
Similar content being viewed by others
References
Akabane A, Kato I, Takasawa S, Unno M, Yonekura H, Yoshimoto T, Okamoto H. Nicotinamide inhibits IRF-1 mRNA induction and prevents IL-1 beta-induced nitric oxide synthase expression in pancreatic beta cells. Biochem Biophys Res Commun 215:524–530;1995.
Bowes J, Thiemermann C. Effects of inhibition of the activity of poly (ADP-ribose) synthase on the liver injury caused by ischemia-reperfusion: A comparison with radical scavengers. Br J Pharmacol 124:1254–1260;1998.
Bowes J, Piper J, Thiemermann C. Inhibitors of the activity of poly(ADP-ribose)synthetase reduce the cell death caused by hydrogen peroxide in human cardiac myoblasts. Br J Pharmacol 124:1760–1766;1998.
Burkart V, Blaeser K, Kolb H. Potential beta-cell protection in vitro by an isoquinolinone-derived PARP inhibitor. Horm Metab Res 31:641–644;1999.
Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest 85:1936–1943;1990.
Cuzzocrea S, Zingarelli B, Caputi AP. Role of peroxynitrite and poly (ADP-ribosyl) synthase activation in cardiovascular derangement induced by zymosan in the rat. Life Sci 63:923–933;1998.
Cuzzocrea S, Zingarelli B, Caputi AP. Peroxynitrite-mediated DNA strand breakage activates poly (ADP-ribose) synthase and causes cellular energy depletion in a nonseptic shock model induced by zymosan in the rat. Shock 9:336–340;1998.
Cuzzocrea S, Costantino G, Zingarelli B, Caputi AP. Protective effect of poly (ADP-ribose) synthase inhibitors in zymosan-activated plasma induced paw edema. Life Sci 65:957–964,1999.
Ducrocq S, Benjelloun N, Plotkine M, Ben-Ari Y, Charriaut-Marlaugue C. Poly(ADP-ribose) synthase inhibition reduces ischemic injury and inflammation in neonatal rat brain. J Neurochem 74:2504–2511;2000.
Endres M, Scott G, Namura S, Salzman AL, Huang PL, Moskowitz MA, Szabo C. Role of peroxynitrite and neuronal nitric oxide synthase in the activation of poly (ADP-ribose) synthetase in a murine model of cerebral ischemia-reperfusion. Neurosci Lett 248:41–44;1998.
Gale EA. Molecular mechanisms of beta-cell destruction in IDDM: The role of nicotinamide. Horm Res 45(suppl 1):39–43;1996.
Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM, Seok JH, Lee JH. Hepatic ischemia/ reperfusion in rats induces iNOS gene transcription by activation of NF-kappa B. Biochem Biophys Res Commun 261:917–922;1999.
Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol 260:G355-G362;1991.
Lentsch AB, Yoshidome H, Kato A, Warner RL, Cheadle WG, Ward PA, Edwards MJ. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology 30:1448–1453;1999.
Liaudet L, Szabo A, Soriano FG, Zingarelli B, Szabo C, Salzman AL. Poly (ADP-ribose) synthase mediates intestinal mucosal barrier dysfunction after mesenteric ischemia. Shock 14:134–141;2000.
Liu P, Xu B, Spokas E, Lai PS, Wong PY. Role of endogenous nitric oxide in TNF-alpha and IL-1beta generation in hepatic ischemia-reperfusion. Shock 13:217–223;2000.
Lo SK, Lee S, Ramos RA, Cobb R, Rosa M, Chi-Rosso G, Wright SD. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 on human neutrophils. J Exp Med 173:1493–1500;1991.
Nakamura K, Ienaga K, Yokozawa T, Fujitsuka N, Oura H. Production of methyl guanidine from creatinine via cretol by active oxygen species: Analysis of the catabolism in vitro. Nephron 58:42–46;1991.
Pearl RG, Raffin TA. Niacin reduces oxygen toxicity in mouse alveolar macrophages. Pharmacology 27:219–222;1983.
Pellat-Deceunynck C, Wietzerbin J, Drapier JC. Nicotinamide inhibits nitric oxide synthase mRNA induction in activated macrophages. Biochem J 297:53–58;1994.
Peralta C, Fernandez L, Panes J, Prats N, Sans M, Pique JM, Gelpi E, Rosello-Catafau J. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced p-selectin up-regulation in the rat. Hepatology 33:100–113;2001.
Remick DG, Colletti LM, Scales WA, McCurry KR, Campbell DA Jr. Cytokines and extrahepatic sequelae of ischemia-reperfusion injury to the liver. Ann NY Acad Sci 723:271–283;1994.
Rosser BJ, Gores GJ. Liver cell necrosis: Cellular mechanisms and clinical implications. Gastroenterology 108:252–275;1995.
Suzuki S, Konno H, Nakamura S. Role of cytokines in hepatic ischemia and reperfusion injury. Nippon Geka Gakkai Zasshi 100:325–330;1999.
Szabo C, Billiar TR. Novel roles of nitric oxide in hemorrhagic shock. Shock 12:1–9;1999.
Thiemermann C, Bowes J, Myint FP, Vane JR. Inhibition of the activity of poly (ADP-ribose) synthase reduces ischemia-reperfusion injury in the heart and skeletal muscle. Proc Natl Acad Sci USA 94:679–683;1997.
Toyoda T, Kassell NF, Lee KS. Attenuation of ischemia-reperfusion injury in the rat neocortex by the hydroxyl radical scavenger nicaraven. Neurosurgery 40:372–377;1997.
Vajragupta O, Toasaksiri S, Boonyarat C, Wongkrajang Y, Peungricha P, Watanabe H, Boonchoong P. Chroman amide and nicotinyl amide derivatives: Inhibition of lipid peroxidation and protection against head trauma. Free Radic Res 32:145–155;2000.
Wang D, Chen HI, Wei J. Effects of nitric oxide synthase inhibitors on systemic hypotension, cytokines and inducible nitric oxide synthase expression and lung injury following endotoxin administration in rats. J Biomed Sci 6:28–35;1999.
Weitberg AB. Effect of nicotinic acid supplement in vivo on oxygen radicals-induced genetic damage in human lymphocytes. Mutat Res 216:197–201;1989.
Zager RA. Adenine nucleotide changes in kidney, liver, and small intestine during different forms of ischemic injury. Circ Res 68:185–196;1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, CF., Wang, D., Hwang, C.P. et al. The protective effect of niacinamide on ischemia-reperfusion-induced liver injury. J Biomed Sci 8, 446–452 (2001). https://doi.org/10.1007/BF02256606
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02256606