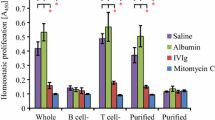
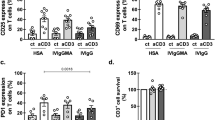
Abstract
Intravenous immunoglobulin (IVIG) has the potential to regulate Ig production, but the mechanism(s) responsible for this effect is unknown. In experiments reported here, we examined the ability of IVIG to regulate Ig production in human peripheral blood mononuclear cells (PBMCs) stimulated with pokeweed mitogen (PWM). IVIG (2–10 mg/ml) showed a potent (80–85%) inhibition of PWM-stimulated IgG, IgM, and IgA production. To determine more precisely how IVIG mediated the inhibition of Ig production, we studied Ig promoting cytokine gene expression after PWM stimulation with or without IVIG (2 and 10 mg/ml) using dot-blot techniques. RNA was isolated from PBMCs at predetermined time points and probed with cDNAs specific for human cytokines (IL-1-β, IL-2, IL-2R, IL-4, IL-5, IL-6, γ-IFN, and TNF-α). IL-6 mRNA accumulation was maximal at 4.5 hr post-PWM stimulation and was inhibited 64–75% when IVIG (10 mg/ml) was present. γ-IFN mRNA levels peaked at 72 hr poststimulation and were also 68–75% inhibited by IVIG. IL-2 mRNA levels peaked at 4.5 hr and were 23–46% inhibited by IVIG. The inhibitory effect of IVIG on production of these cytokines (IL-6 and γ-IFN) was also observed at the protein level in sonicated PBMCs after incubation with PWM and IVIG. The mRNA levels for other cytokines were not or only minimally inhibited by IVIG. Addition of IL-6, γ-IFN, or IL-2 partially restored Ig production in IVIG-treated PWM-stimulated cultures, suggesting that inhibition of other cytokines or another mechanism(s) independent of cytokine inhibition might also be involved, although inhibition of IL-6, γ-IFN, and IL-2 may be one of the critical factors in the suppression of Ig production by IVIG.
Similar content being viewed by others
References
Kaveri SV, Dietrich G, Hurez V, Kazatchkine MD: Intravenous immunoglobulins (IVIg) in the treatment of autoimmune diseases. Clin Exp Immunol 86:192–198, 1991
Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, Tamura T, Hirose O, Manabe Y, Yokoyama T, Kawarano M, Baba K, Mori C: High-dose intravenous gammaglobulin for Kawasaki disease. Lancet II:1055–1057, 1984
Newburger JW, Takahashi M, Burns JC, Beiser AS, Bhung KJ, Duffy DE, Glode MP, Mason WH, Reddy V, Sanders SP, Shulman ST, Wiggins JW, Hicks RV, Julton DR, Lewis AB, Leung DYM, Bolton T, Rosen FS, Melish ME: The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med 315:341–347, 1986
Jayne DRW, Davies MJ, Fox CJV, Black CM, Lockwood CM: Treatment of systemic vasculitis with pooled intravenous immunoglobulin. Lancet 337:1137–1139, 1991
Jordan SC: Intravenous gamma-globulin therapy in systemic lupus erythematosus and immune complex disease. Clin Immunol Immunopathol 53:S164–169, 1989
Sultan Y, Kawatchkine MD, Maisonneuve P, Nydegger UE: Anti-idiotypic suppression of autoantibodies to factor VIII (antihaemophilic factor) by high-dose intravenous gammaglobulin. Lancet II:765–768, 1984
Groothoff JW, Van Leeuwen EF: High dose intravenous gammaglobulin in chonic systemic juvenile arthritis. Br Med J 296:1362–1363, 1988
McGuire WA, Yang HH, Bruno E, Brandt J, Briddell R, Coates TD, Hoffman R: Treatment of antibody-mediated pure red-cell aplasia with high-dose intravenous gamma globulin. N Engl J Med 317:1004–1008, 1987
Lundkvist I, Van Doorn PA, Vermeulen M, Van Lint M, Van Rood JJ, Brand A: Regulation of autoantibodies in inflammatory demyelinating polyneuropathy: Spontaneous and therapeutic. Immunol Rev 110:105–117, 1989
Bussel JB, Cunningham-Rundles C, Abraham C: Intravenous treatment of autoimmune hemolytic anemia with very high dose gammaglobulin. Vox Sang 51:264–269, 1986
Hashimoto F, Sakiyama Y, Matsumoto S: The suppressive effect of gammaglobulin preparations on in vitro pokeweed mitogen-induced immunoglobulin production. Clin Exp Immunol 65:409–415, 1986
Stohl W: Modulation of the immune response by immunoglobulin for intravenous use. I. Inhibition of pokeweed mitogen-induced B cell differentiation. Clin Exp Immunol 62:200–207, 1985
Durandy A, Fischer A, Griscelli C: Dysfunctions of pokeweed mitogen-stimulated T and B lymphocyte responses induced by gammaglobulin therapy. J Clin Invest 67:867–877, 1981
Ballow M, White W, Desbonnet C: Modulation of in vitro synthesis of immunoglobulin and the induction of suppressor activity by therapy with intravenous immune globulin. J Allergy Clin Immunol 84:595–602, 1989
Kondo N, Ozawa T, Mushiake K, Motoyoshi F, Kameyama T, Kasahara K, Kaneko H, Yamashina M, Kato Y, Orii T: Suppression of immunoglobulin production of lymphocytes by intravenous immunoglobulin. J Clin Immunol 11:152–158, 1991
O'Garra A, Umland S, De France T, Chistiansen J: B-cell factors are pleiotropic. Immunol Today 9:45–54, 1988
Lemire JM, Adams JS, Sakai R, Jordan SC: 1a,25-Dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest 74:657–661, 1984
Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA: Nucleotide sequence of human monocyte interleukin 1 precusor cDNA. Proc Natl Acad Sci USA 81:7907–7911, 1984
Holbrook NJ, Smith KA, Jornace AJ, Comeau CM, Wiskocil RL, Crabtree GR: T-cell growth factor: Complete nucleotide sequence and organization of the gene in normal and malignant cells. Proc Natl Acad Sci USA 81:1634–1638, 1984
Leonard WJ, Depper JM, Crabtree GR, Rudikoff S, Pumphey J, Robb RJ, Kronke M, Svetlik PB, Peffer NJ, Waldmann TA, Greene WC: Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature 311:626–631, 1984
Yokota T, Otsuka T, Mosmann T, Bancherear J, Defrance T, Blanchard D, De Vries JE, Lee F, Arai K: Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci USA 83:5894–5898, 1986
Yokota T, Coffman RL, Hagiwara H, Rennick DM, Takebe Y, Yokota K, Gemmell L, Shader B, Yang G, Meyerson P, Luh J, Hoy P, Pene J, Briere P, Spits H, Banchereau J, De Vries J, Lee JD, Arai N, Arai K: Isolation and characterization of lymphokine cDNA clones encoding mouse and human IgA-enhancing factor and eosinophil colony-stimulating factor activities: Relationship to interleukin 5. Proc Natl Acad Sci USA 84:7388–7392, 1987
Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, Tsunasawa S, Sakiyama F, Matsui H, Takahara Y, Taniguchi T, Kishimoto T: Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 324:73–76, 1986
Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, Koh WJ, Aggarwal BB, Goeddel DV: Human tumour necrosis factor: Precursor structure, expression and homology to lymphotoxin. Nature 312:724–729, 1984
Gray PW, Leung DW, Pennica D, Yelverton E, Najarian R, Simonsen C, Derynck R, Sherwood PJ, Wallace DM, Berger SL, Levinson AD, Goeddel DV: Expression of human immune interferon cDNA in E. coli and monkey cells. Nature 295:503–508, 1982
Huck S, Fort P, Crawford DH, Lefranc MP, Lefranc G: Sequence of a human immunoglobulin gamma 3 heavy chain constant region gene: Comparison with the other human Cg genes. Nucleic Acid Res 14:1779–1789, 1986
Gunning P, Ponte P, Okayama H, Engel J, Blau H, Kedes L: Isolation and characterization of full-length cDNA clones for human a-, b-, and g-actin mRNAs: Skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol 3:787–795, 1983
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159, 1987
White BA, Bancroft C: Cytoplasmic dot hybridization. J Biol Chem 257:8569–8572, 1982
Thomas PS: Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA 77:5201–5205, 1980
Church GM, Gilbert W: Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995, 1984
Feinberg AP, Bogelstein B: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13, 1984
Feinberg AP, Bogelstein B: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity: Addendum. Anal Biochem 137, 266–267, 1984
Kirk RR:In Experimental Design: Procedures for the Behavioral Sciences, 2nd ed. Belmont, CA, Wadsworth, 1982, pp 106–114
Schiff RI: Half-life and clearance of pH 6.8 and pH 4.25 immunoglobulin G intravenous preparations in patients with primary disorders of humoral immunity. Rev Infect Dis 8 (Suppl 4):S449-S456, 1986
Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA, Wedgwood RJ: The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med 112:634–640, 1988
Moretta L, Webb SR, Grossi CE, Lydyard PM, Cooper MD: Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med 146:184–200, 1977
Bich-Thuy LT, Brochier J: Human B cell differentiation. II. Suppression by T cells of T-dependent and T independent plasma cell maturation. J Immunol 122:1842–1848, 1979
Kashiwa H, Wright SC, Bonavida B: Regulation of B cell maturation and differentiation. I. Suppression of pokeweed mitogen-induced B cell differentiation by tumor necrosis factor (TNF). J Immunol 138:1383–1390, 1987
Bonavida B, Wright S, Kashiwa H, Graves S, Safrit J, Baldwen RL, Wisnieski B, Bramhall J: Immunoregulation and cytotoxicity by tumor necrosis factor.In Tumor Necrosis Factor/Cachectin and Related Cytokines, Bonavida, Gifford, Kirchner, Old (eds). Basel, Karger, 1988, pp 53–58
Stohl W: Cellular mechanisms in the in vitro inhibition of pokeweed mitogen-induced B cell differentiation by immunoglobulin for intravenous use. J Immunol 136:4407–4413, 1986
Keightley RG, Cooper MD, Lawton AR: The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol 117:1538–1544, 1976
Rosenberg SA, Lipsky PE: The role of monocytes in pokeweed mitogen-stimulated human B cell activation: Separate requirements for intact monocytes and a soluble monocyte factor. J Immunol 126:1341–1345, 1981
Rosenberg SA, Lipsky PE: Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol 122:926–931, 1979
Muraguchi A, Hirano T, Tang B, Matsuda T, Horii Y, Nakajima K, Kishimoto T: The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med 167:332–344, 1988
Leibson HJ, Gefter M, Zlotnik A, Marrack P, Kappler JW: Role of g-interferon in antibody-producing responses. Nature 309:799–801, 1984
Sidman CL, Marshall JD, Shults LD, Gray PW, Johnson HM: g-Interferon is one of several direct B cell-maturing lymphokines. Nature 309:801–804, 1984
Parker DC: Separable helper factors support B cell proliferation and maturation to Ig secretion. J Immunol 129:469–474, 1982
Leibson HJ, Marrack P, Kappler J: B cell helper factors. II. Synergy among three helper factors in the response of T cell- and macrophage-depleted B cells. J Immunol 129:1398–1402, 1982
Leung DM: Immunomodulation by intravenous immune globulin in Kawasaki disease. J Allergy Clin Immunol 84:588–594, 1989
Horii Y, Muraguchi A, Iwano M, Matsuda T, Hirayama T, Yamada H, Fujii Y, Kohi K, Ishikawa H, Ohmoto Y, Yoshizaki K, Hirano T, Kishimoto T: Involvement of IL-6 in mesangial proliferative glomerulonephitis. J Immunol 143:3949–3955, 1989
Linker-Israeli M, Deans RJ, Wallace JD, Prehn J, Ozeri-Chen T, Klinenberg JR: Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol 147:117–123, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toyoda, M., Zhang, X., Petrosian, A. et al. Modulation of immunoglobulin production and cytokine mRNA expression in peripheral blood mononuclear cells by intravenous immunoglobulin. J Clin Immunol 14, 178–189 (1994). https://doi.org/10.1007/BF01533367
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01533367