Abstract
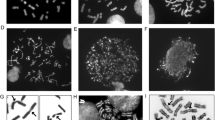
In situ nick translation allows the detection of DNase I sensitive and insensitive regions in fixed mammalian mitotic chromosomes. We have determined the difference in DNase I sensitivity between the active and inactive X chromosomes inMicrotus agrestis (rodent) cells, along both their euchromatic and constitutive heterochromatic regions. In addition, we analysed the DNase I sensitivity of the constitutive heterochromatic regions in mouse chromosomes. InMicrotus agrestis female cells the active X chromosome is sensitive to DNase I along its euchromatic region while the inactive X chromosome is insensitive except for an early replicating region at its distal end. The late replicating constitutive heterochromatic regions, however, in both the active and inactive X chromosome are sensitive to DNase I. In mouse cells on the other hand, the constitutive heterochromatin is insensitive to DNase I both in mitotic chromosomes and interphase nuclei.
Similar content being viewed by others
References
Arrighi FE, Hsu TC, Saunders P, Saunders GF (1970) Localization of repetitive DNA in the chromosomes of microtus agrestis by means of in situ hybridization. Chromosoma 32:224–236
Cooper JE, Hsu TC (1972) The C band and G band patterns of Microtus agrestis chromosomes. Cytogenetics 11:295–304
Gaillard C, Doly J, Cortadas J, Bernardi G (1981) The primary structure of bovine satellite 1.715. Nucl Acids Res 9:6069–6082
Garel A, Axel R (1976) Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci USA 73:3966–3970
Gazit B, Cedar H, Lerer I, Voss R (1982) Active genes are sensitive to Deoxyribonuclease I during metaphase. Science 217:648–650
Gjerset RA, McCarthy BJ (1977) Limited accessibility of chromatin satellite DNA to RNA polymerase from E. coli. Proc Natl Acad Sci USA 74:4337–4340
Goodfellow P, Pym B, Mohandas T, Shapiro LJ (1984) The cell surface antigen locus, MIC2X, escapes X-inactivation. Am J Hum Genet 36:777–782
Holmquist G, Gray M, Porter T, Jordan J (1982) Characterization of Giemsa dark- and light-band DNA. Cell 31:121–129
Hsu TC (1962) Differential rate in RNA synthesis between euchromatin and heterochromatin. Exp Cell Res 27:332–334
Kaput J, Sneider TW (1979) Methylation of somatic vs germ cell DNA analyzed by restriction endonuclease digestions. Nucleic Acids Res 7:2303–2322
Kerem B, Goitein R, Richler C, Marcus M, Cedar H (1983) In situ nick translation distinguishes between active and inactive X chromosomes. Nature 5921:89–90
Kerem B, Goitein R, Diamond G, Cedar H, Marcus M (1984) Mapping of DNase I sensitive regions on mitotic chromosomes. Cell 38:493–499
Lee JC, Yunis JJ (1971) Cytological variation in the constitutive heterochromatin of Microtus agrestis. Chromosoma 35:117–124
Lyfschytz E, Hareven D, Azriel A, Brodsly H (1983) DNA clones and RNA transcripts of four lampbrush loops from the Y chromosome of Drosophila hydei. Cell 32:191–199
Marcus M, Nielsen K, Goitein R, Gropp A (1979) Pattern of condensation of mouse and Chinese-hamster chromosomes in G2 and mitosis in 33258-Hoechst treated cells. Exp Cell Res 122:191–201
Pardue ML, Gall JG (1970) Chromosomal localization of mouse satellite DNA. Science 168:1356–1358
Salmon R, Kaye AM, Herzgerg M (1969) Mouse nuclear satellite DNA: 5-methylcytosine content, pyrimidine isoplith distribution and electron microscopic appearance. J Mol Biol 43:581–592
Schmid W (1967) Heterochromatin in mammals. Arch. Klaus. Stift. Vererb-Forsch 42:1–60
Sieger M, Pera F, Schwarzacher HG (1970) Genetic inactivity of heterochromatin and heteropycnosis in Microtus agrestis. Chromosoma 29:349–364
Sperling K (1982) Cell cycle and chromosome cycle: morphological and functional aspects. In: Rao PN, Johnson RT, Sperling K (eds) Premature chromosome condensation. Academic Press, New York
Sperling K, Rao PN (1974) Mammalian cell fusion. Chromosoma 45:121–131
Stalder J, Groudine M, Dodgson JB, Engel JD, Weintraub H (1980) Hb switching in chickens. Cell 19:973–980
Varley JM, Macgregor HC, Erba HP (1980) Satellite DNA is transcribed on lampbrush chromosomes. Nature 283:686–689
Weintraub H, Groudine M (1976) Chromosomal subunits in active genes have an altered conformation. Science 193:848–856
Wolf U, Flinspack G, Bohm R, Ohno S (1965) DNS Reduplikationsmuster bei den Riesen Geschlechtschromosomen von Micotus agrestis. Chromosoma 16:609–617
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sperling, K., Kerem, B.S., Goitein, R. et al. DNase I sensitivity in facultative and constitutive heterochromatin. Chromosoma 93, 38–42 (1985). https://doi.org/10.1007/BF01259444
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01259444