Abstract
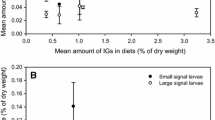
The potential role of iridoid glycosides as feeding stimulants forEuphydryas chalcedona larvae was examined in three laboratory experiments. The first experiment examined larval behavior in choice tests between an artificial diet with no additives (AD) and an artificial diet with the iridoid glycoside, catalpol, added (AD + I) in one group; and AD and AD plus a crude extract from which the iridoid glycoside catalpol was crystallized (AD + Ex) in the second group. The larvae were found more often on AD + I or AD + Ex. The second experiment quantified larval consumption of artificial diets when given a choice of AD or AD + I, and AD or AD + Ex, and showed that larvae ate significantly more AD + I or AD + Ex than AD. The third experiment compared growth and survival on six diets: AD; AD + I; artificial diet with dried, ground upScrophularia californica leaves (AD + S); artificial diet with dried, ground upPlantago lanceolata leaves (AD + P);S. californica leaves (S); andP. lanceolata leaves (P). Growth was best onS. californica leaves, and survival was highest onS. californica andP. lanceolata leaves. There were no differences in growth rate or survival between AD andAD + I. Thus, iridoid glycosides serve as feeding attractants and stimulants for larvae ofEuphydryas chalcedona and are suggested as the basis of radiation in butterflies of the genusEuphydryas.
Similar content being viewed by others
References
Abdusamatov, A., andYusunov, S.Y. 1971. Pediculinine: A new alkaloid fromPedicularis olgae.Khim. Prir. Soedin. 7:306–309 (Chem. Abst.).
Bernays, E., andDeLuca C. 1981. Insect anti-feedant properties of an iridoid glycoside: Ipolamiide.Experientia 37:1289–1290.
Bobbitt, J.M., andSegebarth, K.P. 1969. Iridoid glycosides and similar substances, pp. 1–145,in W.I. Taylor and A.R. Battersby (eds.). Cyclopentanoid Terpene Derivatives. Marcel Dekker Inc., New York.
Bowe, J.J. 1972. Another larval foodplant forEuphydryas phaeton (Drury) (Nymphalidae).J. Lepid. Soc. 26:122.
Bowers, M.D. 1980. Unpalatability as a defense strategy ofEuphydryas phaeton (Lepidoptera: Nymphalidae).Evolution 34:586–600.
Bowers, M.D. 1981. Unpalatability as a defense strategy of western checkerspot butterflies (Euphydryas Scudder, Nymphalidae).Evolution 35:367–375.
Brower, L.P., andBrower, J.V.Z. 1964. Birds, butterflies and plant poisons: A study in ecological chemistry.Zoologica 49:137–159.
Brown, I.L., andEhrlich, P.R. 1980. Population biology of the checkerspot butterfly,Euphydryas chakedona: Structure of the Jasper Ridge colony. Oecologia 47:239–251.
Chew, F.S. 1977. Coevolution of pierid butterflies and their cruciferous foodplants. II. Distribution of eggs on potential foodplants.Evolution 31:568–579.
Chew, F.S. 1980. Foodplant preferences ofPieris caterpillars (Lepidoptera).Oecologia 46:347–353.
Clark, A.H. 1927. Notes on the melitaeid butterflyEuphydryas phaeton (Drury) with descriptions of a new subspecies and a new variety.Proc. U.S. Natl. Mus. Wash. 71 (article no. 2683), 1–22.
Cullenward, M.J., Ehrlich, P.R., White, R.R., andHoldren, C.E. 1979. The ecology and population genetics of an alpine checkerspot butterfly,Euphydryas anicia.Oecologia 38:1–12.
David, W.A.L., andGardner, B.O.C. 1966a. The effect of sinigrin on the feeding ofPieris brassicae L.: Larvae transferred from various diets.Entomol. Exp. Appl. 9:95–98.
David, W.A.L., andGardner, B.O.C. 1966b. Mustard oil glucosides as feeding stimulants forPieris brassicae larvae in a semi-synthetic diet.Entomol. Exp. Appl. 9:247–255.
Dethier, V.G. 1941. Chemical factors determining the choice of food plants byPapilio larvae.Am. Nat. 75:61–73.
Dethier, V.G. 1947. Chemical Insect Attractants and Repellents. Blakiston Co., New York.
Dethier, V.G. 1954. Evolution of feeding preferences in phytophagous insects.Evolution 8:33–54.
Dethier, V.G. 1973. Electrophysiological studies of gustation in lepidopterous larvae II. Taste spectra in relation to food plant discrimination.J. Comp. Physiol. 82:103–134.
Duff, R.B., Bacon, J.S.D., Mundie, C.M., Farmer, V.C., Russell, J.D., andForrester, A.R. 1965. Catalpol and methyl catalpol: Naturally occurring glycosides inPlantago andBuddleia species.Biochem. J. 96:1–5.
Ehrlich, P.R., andRaven, P.H. 1964. Butterflies and plants: A study in coevolution.Evolution 18:586–608.
Ehrlich, P.R., White, R., Singer, M.C., McKechnie, W.W., andGilbert, L.G. 1975. Checkerspot butterflies: A historical perspective.Science 188:221–228.
Feeny, P.P. 1975. Biochemical coevolution between plants and their insect herbivores, pp. 3–19,in L.E. Gilbert and P.H. Raven (eds.). Coevolution of Animals and Plants. Universtiy of Texas Press, Austin.
Fraenkel, G. 1959. The raison d'etre of secondary plant substances.Science 129:1466–1470.
Fraenkel, G. 1969. Evaluation of our thoughts on secondary plant substances.Entomol. Exp. Appl. 12:473–486.
Harbourne, J.B. 1973. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. Chapman and Hall, London.
Higgins, L.G., andRiley, N.D. 1980. A Field Guide to the Butterflies of Britain and Europe. Collins, London.
Jensen, S.R., Nielsen, B.J., andDahlgren, R. 1975. Iridoid compounds, their occurrence and systematic importance in the angiosperms. Bot. Not. 128:148–180.
Jermy, T., Hanson, F.E., andDethier, V.G. 1968. Induction of specific foodplant preference in lepidoptrous larvae.Entomol. Exp. Appl. 11:211–230.
Jirawongse, V. 1964. A chemotaxonomic study of the Scrophulariaceae. PhD thesis, Purdue University, University Microfilms, Inc.
Klots, A.B. 1958. A Field Guide to the Butterflies. Houghton Mifflin Co., Boston.
Kooiman, P. 1970. The occurrence of iridoid glycosides in the Scrophulariaceae.Acta Bot. Neerl. 19:329–340.
Lincoln, D.E. 1980. Leaf resin flavonoids ofDiplacus aurantiacus.Biochem. Syst. Ecol. 8:397–400.
Lincoln, D.E., Newton, T.S., Ehrlich, P.R., andWilliams, K.S. 1982. Coevolution of the checkerspot butterflyEuphydryas chalcedona and its larval food plantDiplacus aurantiacus: Larval response to protein and leaf resin.Oecologia 52:216–223.
Lutfullin, K.L., Yuldashev, P.K., andUnosov, S.Y. 1965. Investigation of the alkaloids ofPedicularis olgae: Structure of plantagonine and indicaine.Khim. Prir. Soedin. Akad. Nauk. USSR 1965(5):365–366.
Ma, W.C., andKubo, I. 1977. Phagostimulants forSpodoptera exempta: Identification of adenosine fromZea mays.Entomol. Exp. Appl. 22:107–112.
Masters, J.H. 1968.Euphydryas phaeton in the Ozarks.Entomol. News 79:85–91.
Morrison, P.D.,Williams, K.S.,Lincoln, D.E., andEhrlich, P.R. 1983. The use of an artificial diet for evaluating larval-hostplant relations ofEuphydryas colon (Nymphalidae). Submitted.
Nayar, J.K., andFraenkel, G. 1963. The chemical basis of host selection in the catalpa sphinx,Ceratomia catalpae (Lepidoptera: Sphingidae).Ann. Entomol. Soc. Am. 56:119–122.
Rausher, M.D. 1982. Population differentiation inEuphydryas editha butterflies: larval adaptation to different hosts.Evolution 36:581–590.
Rodman, J.E., andChew, F.S. 1980. Phytochemical correlates of herbivory in a community of native and naturalized Cruciferae.Biochem. Syst. Ecol. 8:43–50.
Schoonhoven, L.M. 1972. Secondary plant substances and insects, pp. 197–224,in V.C. Runeckles and T.C. Tso (eds.). Recent Advances in Phytochemistry, Vol. 5. Academic Press, New York.
Sevastopolo, D.G. 1964. Lepidoptera ovipositing on plants toxic to their larvae.J. Lepid. Soc. 18:104.
Singer, M.C. 1971. Evolution of food-plant preference in the butterflyEuphydryas editha.Evolution 25:383–389.
Singer, M.C. 1982. Quantification of host specificity by manipulation of oviposition behavior in the butterflyEuphydryas editha.Oecologia 52:224–229.
Singer, M.C., andEhrlich, P.R. 1979. Population dynamics of the checkerspot butterflyEuphydryas editha.Fortschr. Zool. 25:53–60.
Sokal, R.R., andRohlf, F.J. 1969. Biometry. Freeman, San Francisco.
Souzu, I., andMitsuhashi, H. 1969. Structures of iridoids fromLonicera morrowii.Tetrahedron Lett. 32:2725–2728.
Stanton, M.L. 1979. The role of chemotactile stimuli in the oviposition preferences ofColias butterflies.Oecologia 39:79–91.
Straatman, R. 1962. Notes on certain Lepidoptera ovipositing on plants which are toxic to their larvae.J. Lepid. Soc. 16:99–103.
Tabashnik, B.E., Wheelock, H., Rainbolt, J.D., andWatt, W.B. 1981. Individual variation in oviposition preference in the butterfly,Colias eurytheme.Oecologia 50:225–230.
Takino, Y., Koshioka, M., Kawaguchi, M., Miyahara, T., Tanizawa, H., Ishii, Y., Higashino, M., andHayashi, T. 1980. Quantitative determination of bitter components in gentianaceous plants.Planta Med. 38:344–350.
Tietz, H.M. 1972. An Index to the Life Histories of the North American Macrolepidoptera, Allyn Museum of Entomology, Sarasota, Florida.
Torsell, K. 1968. The structures of alkaloids fromPedicularis olgae Regel (Scrophulariaceae) andPlantago indica (P. ramosa) (Plantaginaceae).Acta Chem. Scand. 22:2715–2716.
Trim, A.R., andHill, R. 1952. The preparation and properties of aucubin, asperuloside and some related glycosides.Biochem. J. 50:310–319.
Vershaffelt, E. 1911. The cause determining the selection of food in some herbivorous insects.Proc. Sci. Kon. Akad. Wet. Amsterdam 13:536–542.
Waldbauer, G.P. 1968. The consumption and utilization of food by insects.Recent Adv. Insect Physiol. 5:229–288.
Wieffering, J.H. 1966. Aucubinartige Glucoside (Pseudoindikane) und verwandte Heteroside als systematische Merkmale.Phytochemistry 5:1053–1064.
Wiklund, D. 1981. Generalist vs. specialist oviposition behavior inPapilio machaon (Lepidoptera) and functional aspects on the hierarchy of oviposition preferences.Oikos 36:163–170.
Williams, E.W.,Holdren, C., andEhrlich, P.R. 1983. The life history and ecology ofEuphydryas gilletii Barnes (Nymphalidae). In preparation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bowers, M.D. The role of iridoid glycosides in host-plant specificity of checkerspot butterflies. J Chem Ecol 9, 475–493 (1983). https://doi.org/10.1007/BF00990220
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00990220