Abstract
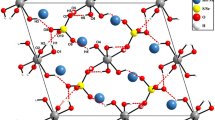
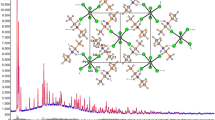
Basic tellurium nitrate crystallizes in the space group Pnma witha=14.607 (1),b=8.801 (1), andc=4.4633 (4) Å. Full-matrix least-squares refinement of automatic diffractometer data converged to a residualR=0.036 (R w =0.046) for 899 independent reflections. A detailed analysis of the structural data leads us to reformulate this compound as (Te2O4H)+(NO3)− with a basic structural element consisting of a charged two-dimensional puckered Te2O4H+ network with discrete NO3 − anions, an example of apositively-charged network structure.
Zusammenfassung
Basisches Telluriumnitrat kristallisiert in der Raumgruppe Pnma mita=14,607 (1),b=8,801 (1) undc=4,4633 (4) Å. Die verfeinernde Auswertung der Diffraktometerdaten konvergierte zuR=0,036 (R w =0.046) für 899 unabhängige Reflexe. Eine detaillierte Analyse der Strukturdaten führte zu einer Umformulierung dieser Verbindung als (Te2O4H)+(NO3)−, wobei die Basisstruktur aus einem geladenen, zweidimensionalen, gefalteten Te2O4H+ Netzwerk mit getrennten NO3 − Anionen besteht. Es stellt dies ein Beispiel einerpositiv geladenen Netzstruktur dar.
Similar content being viewed by others
References
A. F. Wells, private communication.
J.-O. Bovin, Acta Crystallogr.B32, 1771 (1976).
S. Furuseth, K. Selte, H. Hope, A. Kjekshus, andB. Klewe, Acta Chem. Scand.A28, 71 (1974).
G. B. Johansson andO. Lindqvist, Acta Crystallogr.B32, 2720 (1976).
H. Mayer, Z. Kristallogr.141, 354 (1975).
H. Mayer andG. Pupp, Z. Kristallogr.145, 321 (1977).
L. N. Swink andG. B. Carpenter, Acta Crystallogr.21, 578 (1966).
W. R. Busing, K. O. Martin, andH. A. Levy, Oak Ridge National Laboratory, ORNL-TM-305, Oak Ridge, Tennessee, 1962.
D. T. Cromer andJ. B. Mann, Acta Crystallogr.A24, 321 (1968).
W. H. Zachariasen, Acta Crystallogr.23, 558 (1967);B 24, 324 (1968).
J. Galy, G. Meunier, S. Andersson, andA. Åström, J. Solid State Chem.13, 142 (1975).
A. Leclair, J. Solid State Chem.28, 235 (1979).
R. A. Nyquist andR. O. Kagel, Infrared Spectra of Inorganic Compounds, p. 144. New York: Academic Press. 1971.
B. M. Gatehouse, S. E. Livingston, andR. S. Nyholm, J. Chem. Soc.1957, 4222.
I. D. Brown, J. Solid State Chem.11, 214 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anderson, J.B., Rapposch, M.H., Anderson, C.P. et al. Crystal structure refinement of basic tellurium nitrate: A reformulation as (Te2O4H)+(NO3)− . Monatshefte für Chemie 111, 789–796 (1980). https://doi.org/10.1007/BF00899243
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00899243