Abstract
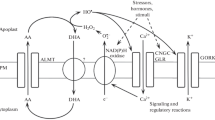
By using lycorine, a specific inhibitor of ascorbate biosynthesis, it was possible to demonstrate that plant cells consume a high quantity of ascorbate (AA). Thein vivo metabolic reactions utilizing ascorbate are the elimination of H2O2 by ascorbate peroxidase and the hydroxylation of proline residues present in the polypeptide chains by means of peptidyl-proline hydroxylase.
Ascorbate acts in the cell metabolism as an electron donor, and consequently ascorbate free radical (AFR) is continuously produced. AFR can be reconverted to AA by means of AFR reductase or can undergo spontaneous disproportion, thus generating dehydroascorbic acid (DHA).
During cell division and cell expansion ascorbate consumption is more or less the same; however, the AA/DHA ratio is 6–10 during cell division and 1–3 during cell expansion. This ratio depends essentially on the different AFR reductase activity in these cells. In meristematic cells AFR reductase is very high, and consequently a large amount of AFR is reduced to AA and a small amount of AFR undergoes disproportionation; in expanding cells the AFR reductase activity is lower, and therefore AFR is massively disproportionated, thus generating a large quantity of DHA. Since the transition from cell division to cell expansion is marked by a large drop of AFR reductase activity in the ER, it is suggested here that AFR formed in this compartment may be involved in the enlargement of the ER membranes and provacuole acidification.
DHA is a toxic compound for the cell metabolism and as such the cell has various strategies to counteract its effects: (i) meristematic cells, having an elevated AFR reductase, prevent large DHA production, limiting the quantity of AFR undergoing disproportionation. (ii) Expanding cells, which contain a lower AFR reductase, are, however, provided with a developed vacuolar system and segregate the toxic DHA in the vacuole. (iii) Chloroplast strategy against DHA toxicity is efficient DHA reduction to AA using GSH as electron donor. This strategy is usually poorly utilized by the surrounding cytoplasm.
DHA reduction does play an important role at one point in the life of the plant, that is, during the early stage of seed germination. The dry seed does not store ascorbate, but contains DHA, and several DHA-reducing proteins are detectable. In this condition, DHA reduction is necessary to form a limited AA pool in the seed for the metabolic requirements of the beginning of germination. After 30–40h ascorbateex novo synthesis starts, DHA reduction declines until a single isoform remains, as is typical in the roots, stem, and leaves of seedlings. Finally, DHA recycling also appears to be important under adverse environmental conditions and ascorbate deficiency.
Similar content being viewed by others
References
Amico, A., Dalessandro, G., Stefanizzi, L., and De Leo, P. (1972).Ann. Fac. Agr. 25 1–11.
Arrigoni, O. (1957).Rend. Acc. Naz. Lincei 22 77–85.
Arrigoni, O., Rossi, G., and Marrè, E. (1957).Rend. Acc. Naz. Lincei 23 287–295.
Arrigoni, O. (1958).Rend. Acc. Naz. Lincei 19 156–164.
Arrigoni, O., Arrigoni-Liso, R., and Calabrese, G. (1975).Nature (London) 256 513–514.
Arrigoni, O., Arrigoni-Liso, R., and Calabrese, G. (1976).Science 194 332–333.
Arrigoni, O., Arrigoni-Liso, R., and Calabrese, G. (1977a).FEBS Lett. 82 131–138.
Arrigoni, O., De Santis, A., Arrigoni-Liso, R., and Calabrese, G. (1977b).Biochem. Biophys. Res. Comm. 74 1637.
Arrigoni, O., Calabrese, G., Liso, R., and Porcelli, S. (1977c).Ann. Ist. Sper. Ortic. SA,7 1–14.
Arrigoni, O., Di Pierro, S., and Borraccino, G. (1981).FEBS Lett. 125 242–244.
Arrigoni, O., Bitonti, M. B., Cozza, R., Innocenti, A. M., Liso, R., and Veltri, R. (1989).Caryologia 42 213–216.
Arrigoni, O., De Gara, L., Tommasi, F., and Liso, R. (1992).Plant Physiol. 99 236–238.
Barlow, P. W., and Macdonald, P. D. M. (1973).Proc. R. Soc. B. London 183 385–398.
Bitonti, M. B., Chiappetta, A., Innocenti, A. M., Liso, R., and Arrigoni, O. (1992).New Phytol. 121 577–580.
Blakely, L. M., and Evans, T. A. (1979).Plant Sci. Lett. 14 79–83.
Borsook, H., Davenport, H. W., Jeffreys, C. E. P., and Warner, R. C. (1937).J. Biol. Chem. 117 237–279.
Chrispeels, M. J., Sadawa, D., and Cho, Y. P. (1974).J. Exp. Bot. 25 1157–1166.
Cleland, R. (1968a).Plant Physiol. 43 865–870.
Cleland, R. (1968b).Plant Physiol. 43 1625–1630.
Clowes, F. A. L. (1961).J. Exp. Bot. 12 283–293.
Cooper, J. B., and Varner, J. E. (1983).Plant Physiol. 73 324–328.
Corsi, G., and Avanzi, S. (1970).Caryologia 23 381–394.
Craig, T. A., and Crane, F. L. (1982).Indiana Acad. Sci. 91 150–162.
De Gara, L., Tommasi, F., Liso, R., and Arrigoni, O. (1987).Boll. Soc. It. Biol. Sper. LXIII 551–558.
De Gara, L., and Tommasi, F. (1990).Boll. Soc. It. Biol. Sper. LXVI 953–960.
De Gara, L., Paciolla, C., Liso, R., Stefani, A., and Arrigoni, O. (1991a).J. Plant Physiol. 137 697–700.
De Gara, L., Tommasi, F., Liso, R., and Arrigoni, O. (1991b).Phytochemistry 30 1397–1399.
De Gara, L., De Tullio, M., Paciolla, C., Liso, R., and Arrigoni, O. (1993a). InPlant Peroxidase and Physiology (Welinder, K. G., Rasmussen, S. K., Penel, C., and Greppin, H., eds.), University of Geneva, Geneva, Switzerland, pp. 251–255.
De Gara, L., Paciolla, C., Liso, R., Stefani, A., Blanco, A., and Arrigoni, O. (1993b).J. Plant Physiol. 141 405–409.
De Gara, L., Paciolla, C., Tommasi, F., Liso, R., and Arrigoni, O. (1994).J. Plant Physiol. (in press).
De Leo, P., Dalessandro, G., De Santis, A., and Arrigoni, O. (1972).Plant and Cell Physiol. 14 487–496.
De Tullio, M., Paciolla, C., and De Gara, L. (1993).Boll. Soc. It. Biol. Sper. LXIX 231–236.
Evidente, A., Cicala, M. R., Randazzo, G., Riccio, R., Calabrese, G., Liso, R., and Arrigoni, O. (1983).Phytochemistry 22 2193–2196.
Evidente, A., Arrigoni, O., Liso, R., Calabrese, G., and Randazzo, G. (1986).Phytochemistry 25 2739–2743.
Foerster, G. V., Weis, W., and Standinger, H. (1965).Ann. Chem. 690 166–169.
Forti, G. (1958).Rend. Acc. Naz. Lincei 24 70–77.
Foyer, C. H., and Halliwell, B. (1976).Planta 133 21–25.
Garuccio, I., and Arrigoni, O. (1979).Boll. Soc. It. Biol. Sper. 45 501–508.
Groden, D., and Beck, E. (1979).Biochem. Biophys. Acta 546 426–435.
Hedge, R. R. (1985).Bot. Mag. Tokyo 98 219–223.
Hidalgo, A., Gonzales-Reyes, J. A., and Navas, P. (1989).Plant Cell Environ. 12 455–460.
Hutton, J. J., Tappel, A. L., and Udenfriend, S. (1967).Arch. Biochem. Biophys. 118 231–240.
Innocenti, A. M., Bitonti, M. B., Arrigoni, O., and Liso, R. (1990).New Phytol. 114 507–509.
Innocenti, A. M., Bitonti, B., Mazzucca, S., Liso, R., and Arrigoni, O. (1993).Caryologia 46 1–4.
Innocenti, A. M., Mazzucca, S., Bitonti, B., De Gara, L., Liso, R., and Arrigoni, O. (1994).Plant Physiol. Biochemistry (in press).
Lamport, D. T. A. (1965). The protein component of primary cell walls. InAdvances in Botanical Researches (Preston, R. D., ed.),2 151–218.
Laudi, G. (1955).N. Giorn. Bot. Ital. 62 368–373.
Liso, R., and Calabrese, G. (1974).Phycologia 13 205–208.
Liso, R., and Calabrese, G. (1975).Phycologia 14 9–11.
Liso, R., Arrigoni, O., Stomeo, G., and Porcelli, S. (1977).Ann. Ist. Sper. Ort. SA,7 1–11.
Liso, R., Calabrese, G., Bitonti, M. B., and Arrigoni, O. (1984).Exp. Cell. Res. 150 314–320.
Liso, R., Innocenti, A. M., Bitonti, M. B., and Arrigoni, O. (1988).New Phytol. 110 469–471.
Luster, D. G., and Buckhout, T. J. (1988).Physiol. Plant. 73 339–347.
Marrè, E., and Laudi, G. (1955).Rend. Acc. Naz. Lincei 20 77–82.
Marrè, E., Pece, G., and Forti, G. (1956).Rend. Acc. Naz. Lincei 20 646–652.
Marrè, E., and Arrigoni, O. (1958).Biochem. Biophys. Acta 30 453–457.
May, M. J., and Leaver, C. J. (1993).Plant. Physiol. 103 621–627.
Meister, A. (1992).Biochem. Pharmacol. 44 1905–1915.
Navas, P. (1991). Ascorbate free radical (semidehydro) reductase on plant plasma membrane. InOxidoreduction at the Plasma Membrane: Relation to Growth and Transport. Vol. II (Crane, F. L., Morrè, D. J., and Low, H. E., eds.), CRC Press, Boca Raton, Florida, pp. 111–120.
Pece, G., Laudi, G., and Marrè, E. (1956).Rend. Acc. Naz. Lincei 20 513–518.
Pihlajaniemi, T., Helaakoski, T., Tasanen, K., Myllylä, R., Huhtala, M. L., Koivu, J., and Kivirikko, K. I. (1987).EMBO J. 6 643–649.
Rhoads, R. E., and Udenfriend, S. (1970).Arch. Biochem. Biophys. 139 329–339.
Ridge, I., and Osborne, D. J. (1971).Nature New. Biol. 229 205–208.
Shigeoka, S., Nakano, Y., and Kitaoka, S. (1980).Arch. Biochem. Biophys. 201 121–127.
Stone, N., and Meister, A. (1962).Nature 194 555–557.
Sreekumari, S. B., and Shah, C. K. (1978).Acta Bot. Indica,6 (suppl. 22–31.
Szent Györgyi, A. (1928).Biochem. J. 22 1387–1409.
Thomas, J. E., and Davidson, D. (1982).Caryologia 35 191–203.
Tommasi, F., De Gara, L., Liso, R., and Arrigoni, O. (1987).Boll. Soc. It. Biol. 43 779–786.
Tommasi, F., De Gara, L., Liso, R., and Arrigoni, O. (1990).J. Plant Physiol. 135 766–768.
Trezzi, F. (1956).Rend. Acc. Naz. Lincei 21 1–15.
Wells, W. W., Xu, D. P., Yang, Y., and Rocque, P. A. (1990).J. Biol. Chem. 265 15361–15364.
Yamaguchi, M., and Joslyn, M. A. (1951).Plant Physiol. 26 757–772.
Yamaguchi, K., and Suda, S. (1952).Folia pharmacol. Japan 48 31–32.
Zacheo, G., Liso, R., Bleve, T., Lamberti, F., Perrino, F., and Arrigoni, O. (1981).Nematol. Medit. 9 181–187.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arrigoni, O. Ascorbate system in plant development. J Bioenerg Biomembr 26, 407–419 (1994). https://doi.org/10.1007/BF00762782
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00762782