Abstract
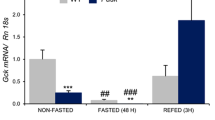
We have studied the role of Glc6Pase mRNA abundance in the time course of Glc6Pase activity in liver and kidney during long-term fasting in rat. Refered to the mRNA level in the fed state, Glc6Pase mRNA abundance was increased by 3.5 ± 0.5 and 3.7 ± 0.5 times (mean ± S.E.M., n = 5) in the 24 h and 48 h-fasted liver, respectively. Then, the liver Glc6Pase mRNA was decreased to the level of the fed liver after 72 and 96 h of fasting (1.0 ± 0.3 and 1.4 ± 0.3). In the kidney, Glc6Pase mRNA abundance was increased by 2.7 ± 1.0 and 5 ± 1.2 times at 24 and 48 h of fasting, respectively. Then, it plateaued at the level of the 48 h fasted kidney after 72 h and 96 h of fasting (4.5 ± 1.0 and 4.3 ± 1.0). After 24 and 48 h-refeeding, the abundance of Glc6Pase mRNA in 48 h-fasted rats was decreased to the level found in the liver and kidney of fed rats. The time course of the activity of Glc6Pase catalytic subunit during fasting and refeeding was strikingly parallel to the time course of Glc6Pase mRNA level in respective tissues. These data strongly suggest that the differential expression of Glc6Pase activity in liver and kidney in the course of fasting may be accounted for by the respective time course of mRNA abundance in both organs.
Similar content being viewed by others
Abbreviations
- Glc6Pase:
-
Glucose-6 phosphatase
- GNG:
-
Gluconeogenesis
References
Hers HG, Hue L: Gluconeogenesis and related aspects of glycolysis. Ann Rev Biochem 52: 617–653, 1983
Alleyne GAO, Scullard GH: Renal metabolic response to acid base changes. Enzymatic control of ammoniagenesis in the rat. J Clin Invest 48: 364–370, 1969
Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF: Liver and kidney metabolism during prolonged starvation. J Clin Invest 48: 574–583, 1969
Minassian C, Mithieux G: Differential time course of liver and kidney glucose-6 phosphatase activity during fasting in rats. Comp Biochem Physiol 109B: 99–104, 1994
Shelly LL, Lei KJ, Pan CJ, Sakata SF, Ruppert S, Schulz G, Chou JY: Isolation of the gene for murine glucose-6 phosphatase, the enzyme deficient in glycogen storage disease type IA. J Biol Chem 268: 21482–21485, 1993
Lei KJ, Shelly LL, Pan CJ, Sidbury JB, Chou JY: Mutations in the glucose-6 phosphatase gene that cause glycogen storage disease type 1a. Science 262: 580–583, 1993
Lange A, Argaud D, El-Maghrabi MR, Pan W, Maitra SR, Pilkis SJ: Isolation of a cDNA clone for the catalytic subunit of rat liver glucose6 phosphatase: regulation of gene expression in FAO hepatoma cells by insulin, dexamethasone and cAMP. Biochem Biophys Res Commun 201: 302–309, 1994
Haber BA, Chin S, Chuang E, Buikhuisen W, Naji A, Taub R: High levels of glucose-6 phosphatase gene and protein expression reflect an adaptative response in proliferating liver and diabetes. J Clin Invest 95: 832–841, 1995
Mithieux G, Vega F, Riou JP: The liver glucose-6 phosphatase of intact microsomes is inhibited and displays sigmoid kinetics in the presence of α-ketoglutarate-magnesium and oxaloacetate-magnesium chelates. J Biol Chem 265: 20364–20368, 1990
Sambrook J, Fritsch EF, Maniatis T: Molecular cloning. A laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press, New York, 1989
Pilkis SJ, Riou JP, Claus TH: Hormonal control of [14C]-glucose synthesis from [U-14C]dihydroxyacetone and glycerol in isolated hepatocytes. J Biol Chem 251: 7841–7852, 1976
Minassian C, Ajzannay A, Riou JP, Mithieux G: Investigation of the mechanism of glycogen rebound in the liver of 72 h. fasted rats. J Biol Chem 269: 16585–16588, 1994
Burchell A, Cain DI: Rat hepatic microsomal glucose-6 phosphatase protein levels are increased in streptozotocin-induced diabetes. Diabetologia 28: 852–856, 1985
Liu Z, Barrett EJ, Dalkin AC, Zwart AD, Chou JY: Effect of acute diabetes on rat hepatic glucose-6 phosphatase activity and its messenger RNA level. Biochem. Biophys Res Commun 205: 680–686, 1994
Garces LY, Kenny FM, Drash A, Taylor FH: Cortisol secretion rate during fasting in obese adolescent subjects. J Clin Endocr 28: 1843–1847, 1968
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Minassian, C., Zitoun, C. & Mithieux, G. Differential time course of liver and kidney glucose-6 phosphatase activity during long-term fasting in rat correlates with differential time course of messenger RNA level. Mol Cell Biochem 155, 37–41 (1996). https://doi.org/10.1007/BF00714331
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00714331