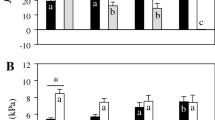
Summary
-
1.
A method is described for the perfusion of the thoracic ganglia of crabs (Cancer, Pugettia, Portunus, Callinectes, Eriphia) through the sternal artery.
-
2.
Administration of acetylcholine (ACh)via perfusion elicits leg movements. The effect is enhanced by the anticholinesterase eserine. Eserine alone causes great enhancement of reflex activity.
-
3.
Eserinized perfusate collected during periods of sensory stimulation (optical or tactile stimuli) contained ACh as detected by bioassay on isolated mollusc ventricles. Up to 2 × 10−9 g of ACh were liberated per minute. No ACh was detectable in perfusates collected from quiescent, unstimulated crabs (detection limit 1−10 × 10−11g/ml) or from stimulated crabs when the perfusion fluid contained no eserine. Eserine itself had no effect on the heart preparations used.
-
4.
The demonstration of a release of ACh from the central nervous system of crabs during sensory stimulation represents the last missing link in the evidence (which is fully reviewed) that ACh is the transmitter substance of sensory neurons in decapod crustacea and, presumably, in other arthropod groups as well.
Zusammenfassung
-
1.
Es wird eine Methode beschrieben, die es erlaubt, die Thorakalganglien von Krabben (Cancer, Pugettia, Portunus, Callinectes, Eriphia) über die Sternal-Arterie zu perfundieren.
-
2.
Applikation von Azetylcholinvia Perfusion ruft Beinbewegungen hervor. Diese Wirkung wird verstärkt durch Eserin (Cholinesterasehemmer). Eserin selbst bewirkt eine erhebliche Verstärkung der Reflexaktivität.
-
3.
Eserinisiertes Perfusat, das während Perioden optischer oder mechanischer Reizung gewonnen wurde, enthielt ACh, das im Biotest an isolierten Ventrikeln von Mollusken nachgewiesen werden konnte. Bis zu 2 × 10−9 g ACh wurden pro Minute freigesetzt. In Perfusaten, die von ungereizten, ruhenden Krabben oder von gereizten Krabben erhalten wurden, wenn die Perfusionsflüssigkeit kein Eserin enthielt, konnte kein ACh nachgewiesen werden (Nachweisgrenze l −10 × 10−11 g/ml).
-
4.
Der Nachweis der Freisetzung von ACh im Zentralnervensystem von Krabben während sensorischer Reizung liefert das letzte noch fehlende Glied in der Beweisführung (die in der Arbeit ausführlich dokumentiert wird), daß ACh die Transmitter-Substanz der sensorischen Neurone der dekapoden Krebse, und vermutlich auch der anderen Arthropodengruppen, darstellt.
Similar content being viewed by others
References
Barker, D. L., Herbert, E., Hildebrand, J. G., Kravitz, E. A.: Acetylcholine and lobster sensory neurones. J. Physiol. (Lond.), in press (1972).
Birks, R., Macintosh, F. C.: Acetylcholine metabolism of a sympathetic ganglion. Canad. J. Biochem.39, 787–827 (1961).
Bonnet, V.: Contribution à l'étude du système nerveux ganglionnaire des crustacés. Arch. int. Physiol.47, 397–433 (1938).
Bullock, T. H., Grundfest, H., Nachmansohn, D., Rothenburg, M. A.: Effect of di-isopropyl fluorphosphate (DFP) on action potential and cholinesterase of nerve, II. J. Neurophysiol.10, 63–78 (1947).
Callec, J., Boistel, J.: Les effects de l'acétylcholine aux niveaux synaptique et somatique dans le cas dernier ganglion abdominal de la Blatte,Periplaneta americana. C. R. Soc. Biol. (Paris)161, 157–181 (1967).
Case, J.: Properties of the dactyl chemoreeeptors ofCancer antennarius Stimpson andC. productus Randall. Biol. Bull.127, 428–446 (1964).
Chiang, P. K.: Some pharmacological properties of the nerve cord of the cockroach,Periplaneta americana (L.). Questiones entomol.5, 263–306 (1969).
Colhoun, E. H.: The physiological significance of acetylcholine in insects and observations upon other pharmacologically active substances. Adv. Insect Physiol.1, 1–46 (1963).
Easton, D. M.: Synthesis of acetylcholine in crustacean nerve and nerve extract. J. biol. Chem.185, 813–816 (1950).
Ellis, C. H., Thienes, C. H., Wiersma, C. A. G.: The influence of certain drugs on the crustacean nerve-muscle system. Biol. Bull.83, 334–352 (1942).
Evoy, W., Beránek, R.: Pharmacological localization of excitatory and inhibitory synaptic regions in crayfish slow abdominal flexor muscle-fibres. Comp. gen. Pharmac.3, 178–186 (1972).
Fatt, P., Katz, B.: The electrical properties of crustacean muscle fibres. J. Physiol. (Lond.)120, 171–204 (1953).
Elattum, R. F., Friedman, S., Larsen, J. R.: The effects of d-tubocurare chloride on nervous activity and muscular contraction in the house cricketAcheta domesticus (L.). Life Sci.6, 1–9 (1967).
Florey, E.: Comparative neurochemistry: inorganic ions, amino acids and possible transmitter substances of invertebrates. In: Neurochemistry, 2nd ed., p. 673–693, ed. Elliott, K. A. C., I. H. Page and J. H. Quastel. Springfield, Ill.: C. C. Thomas 1962.
Florey, E.: Acetylcholine in invertebrate nervous systems. Canad. J. Biochem. Physiol.41, 2619–2626 (1963).
Florey, E.: The clam-heart bioassay for acetylcholine. Comp. Biochem. Physiol.20, 365–377 (1967).
Florey, E., Biederman, M. A.: Studies on the distribution of Factor I and acetylcholine in crustacean peripheral nerve. J. gen. Physiol.43, 509–522 (1960).
Futamachi, K. J.: Acetylcholine: possible neuromuscular transmitter in Crustacea. Science172, 1373–1375 (1972).
Gardiner, J. E.: The inhibition of acetylcholine synthesis in brain by hemicholinium. Biochem. J.81, 297–303 (1961).
Hichar, J. K.: Spontaneous electrical activity in the crayfish central nervous system. J. cell. comp. Physiol.55, 195–206 (1960).
Katz, B.: Neuromuscular transmission in crabs. J. Physiol. (Lond.)87, 199–221 (1936).
Kerkut, G. A., Pittman, R. M., Walker, R. J.: Iontophoretic application of acetylcholine and GABA onto insect central neurones. Comp. Biochem. Physiol.31, 611–633 (1969).
Keyl, M. J., Michaelson, I. A., Whittaker, V. P.: Physiologically active choline esters in certain marine gastropods and other invertebrates. J. Physiol. (Lond.)139, 434–454 (1957).
Knowlton, F. P.: The action of certain drugs on crustacean muscle. J. Pharmacol. exp. Ther.75, 154–160 (1942).
Larsen, J. R., Miller, D. A., Yamamoto, T.: d-Tubocurarine chloride: Effect on insects. Science152, 225–226 (1966).
Marnay, A., Naohmansohn, D.: Cholinestérase dans le nerf de homard. C. R. Soc. Biol. (Paris)125, 1005 (1937).
Maynard, E. A.: Microscopic localization of cholinesterases in the nervous systems of the lobsters,Panulirus argus andHomarus americanus. Tissue and Cell3, 215–250 (1971).
McCann, F. V.: Curare as a neuromuscular blocking agent in insects. Science154, 1023–1024 (1966).
McCann, F. V., Reece, R. W.: Neuromuscular transmission in insects: effect of injected chemical agents. Comp. Biochem. Physiol.21, 115–124 (1967).
Prosser, C. L.: Action potentials in the nervous system of the crayfish. J. cell. comp. Physiol.16, 25–38 (1940).
Richards, A. G., Cutkomp, L. K.: The cholinesterase of insect nerves. J. cell. comp. Physiol.26, 57–61 (1945).
Riker, W. F., Okamoto, M.: Pharmacology of motor nerve terminals. Pharmacol. Rev.9, 173–208 (1969).
Smallman, B. N.: Mechanism of acetylcholine synthesis in the blowfly. J. Physiol. (Lond.)132, 343–357 (1956).
Sorenson, A. L.: Demonstration of an action of acetylcholine on the central nervous system of a crab. Biol. Bull., in press (1973).
Tobias, J. M., Kollros, J. I., Savit, I.: Acetylcholine and related substances in the cockroach, fly and crayfish and the effect of DDT. J. cell. Comp. Physiol.28, 159–182 (1946).
Treherne, J. E.: The neurochemistry of arthropods. 156 pp. Cambridge: University Press 1966.
Turner, R. S., Hagins, W. A., Moore, A. R.: Influence of certain neurotropic substances on central and synaptic transmission inCallianassa. Proc. Soc. exp. Biol. (N.Y.)73, 156–518 (1950).
Walop, J. N.: Studies on acetylcholine in the crustacean central nervous system. Arch. int. Physiol.59, 145–156 (1951).
Welsh, J. H.: Occurrence of acetylcholine in nervous tissue of crustaceans and its effect on the crab heart. Nature (Lond.)142, 151 (1938).
Welsh, J. H.: Chemical mediation in crustaceans. I. The occurrence of acetylcholine in nervous tissue and its action on the decapod heart. J. exp. Biol.16, 198–219 (1939).
Welsh, J. H., Taub, R.: The action of acetylcholine antagonists on the heart ofVenus mercenaria. Brit. J. Pharmacol.8, 327–333 (1953).
Wiersma, C. A. G., Pilgrim, R. L. C.: Thoracic stretch receptors in crayfish and rock lobsters. Comp. Biochem. Physiol.2, 51–64 (1961).
Wiersma, C. A. G., Furshpan, E., Florey, E.: Physiological and pharmacological observations on muscle receptor organs of the crayfish,Cambarus clarkii Girard. J. exp. Biol.30, 136–150 (1953).
Wright, E. B.: The action of erythroidin, curare, and chlorobutanol in the crayfish. J. cell. comp. Physiol.33, 301–332 (1949).
Yamasaki, T., Narahashi, T.: Synaptic transmission in the last abdominal ganglion of the cockroach. J. Ins. Physiol.4, 1–13 (1960).
Author information
Authors and Affiliations
Additional information
This study was supported by a grant from the United States Public Health Service, National Institutes of Health, NB-1451, and by a grant from the Deutsche Forschungsgemeinschaft through the Forschergruppe “Biologische Grenzflächen und Spezifität”.
Rights and permissions
About this article
Cite this article
Florey, E. Acetylcholine as sensory transmitter in crustacea. J. Comp. Physiol. 83, 1–16 (1973). https://doi.org/10.1007/BF00694568
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00694568