Summary
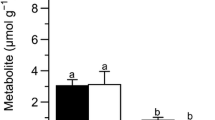
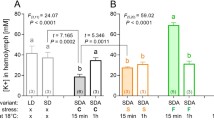
The larvae ofGynaephora groenlandica, a long-lived moth endemic to the high arctic, are perennially freeze-tolerant and able to increase their freeze-tolerance by synthesizing glycerol. Cold-induced mitochondrial changes were correlated (using electron microscopy, DNA staining, cytochrome c assay, and oxygen uptake) with glycerol production (using NMR spectroscopy) in larvae under different acclimations and in the field. Hypometabolism in summer- or warm-acclimated larvae led to glycerol accumulation. Extended exposure to near-zero or freezing temperatures caused mitochondrial degradation and glycerol accumulation. Rapid freezing of warm-acclimated larvae did not result in mitochondrial breakdown. Mitochondrial reconstitution upon warm-acclimation occurred much more rapidly (<1 week) than did degradation (>2 months). Concomitant with mitochondrial breakdown was reduced oxidative metabolism, but the cytochrome c concentration remained independent of acclimation temperature. The adaptive response to cold by mitochondrial degradation and glycerol accumulation byG. groenlandica may be linked to diapause in other species of ectotherms.
Similar content being viewed by others
References
Asahina E (1969) Frost resistance in insects. Adv Insect Physiol 6:1–45
Ballantyne J, Storey KB (1984) Characterization of mitochondria isolated from the freezing-tolerant larvae of the goldenrod gall fly (Eurosta solidaginis): substrate preference, salt effects, and pH effects on warm- and cold-acclimated animals. Can J Zool 63:373–379
Chen C-P, Denlinger DL, Lee RE (1987) Cold-shock injury and rapid cold-hardening in the flesh flySarcophaga crassipalpis. Physiol Zool 60:297–304
Chino H (1957a) Carbohydrate metabolism in diapause egg of the silkworm,Bombyx mori I. Diapause and the change in glycogen content. Embryologia 3:295–316
Chino H (1957b) Conversion of glycogen to sorbitol and glycerol in the diapause egg of theBombyx silkworm. Nature 180:606–607
Chino H (1958) Carbohydrate metabolism in diapause eggs of the silkworm,Bombyx mori. II. Conversion of glycogen into sorbitol and glycerol during diapause. J Insect Physiol 2:1–12
Chino H (1960) Enzymatic pathways in the formation of sorbitol and glycerol in the diapausing eggs of the silkwormBombyx mori. I. On the polyol dehydrogenases. J Insect Physiol 5:1–15
Clarke KU, Baldwin RW (1960) The effect of insect hormones and of 2,4-dinitrophenol on the mitochondria ofLocusta migratoria L. J Insect Physiol 5:37
Colowick SP, Kaplan NO (1955) Methods in enzymology. Academic Press, New York, p 143
Conradi-Larsen E, Somme L (1973) The overwintering ofPelophila borealis Payk. II. Aerobic and anaerobic metabolism. Norsk Ent Tidsskr 20:325–332
Denlinger DL, Willis JH, Fraenkel G (1972) Rates and cycles of oxygen consumption during pupal diapause inSarcophaga flesh flies. J Insect Physiol 18:871–882
Harvey WR, Williams CM (1958) Physiology of insect diapause XII. The mechanisms of carbon monoxide sensitivity and insensitivity during the pupal diapause of the Cecropia silkworm. Biol Bull (Woods Hole, Mass) 114:36–53
Hayakawa Y (1985) Activation mechanism of insect fat body phosphorylase by cold. Insect Biochem 15:123–128
Hayakawa Y, Chino H (1982) Phosphofructokinase as a possible key enzyme regulating glycerol or trehalose accumulation in diapausing insects. Insect Biochem 12:639–642
Herbert D, Phipps RJ, Strange RE (1971) Chemical analysis of microbial cells. Methods Microbiol 5B:209–344
Hochachka PW, Somero GN (1985) Biochemical adaptation. Princeton University Press, Princeton
Ingrisch S (1987) Oxygen consumption by developing and diapausing eggs ofEupholidoptera smyrnensis (Orthoptera: Tetigoniidae). J Insect Physiol 33:861–865
Kageyama T (1976) Pathways of carbohydrate metabolism in the eggs of the silkworm,Bombyx mori. Insect Biochem 6:507–511
Keeley LL (1973) Characterization of insect fat body mitochondria isolated by a rapid procedure. Comp Biochem Physiol 46 B:147–151
Keeley LL (1981) Neuroendocrine regulation of mitochondrial development and function in the insect fat body. In: Downer RGH (ed) Energy metabolism in insects. Plenum Press, New York, pp 207–239
Keister M, Buck J (1964) Respiration. In: Rockstein M (ed) The physiology of Insecta. Academic Press, New York, pp 627–658
Kukal O, Kevan PG (1987) The influence of parasitism on the life history of a high arctic insect,Gynaephora groenlandica (Wocke) (Lepidoptera: Lymantriidae). Can J Zool 65:156–163
Kukal O, Dawson TE (in press) Temperature and food quality influences feeding behavior, assimilation efficiency and growth rate of arctic woolly-bear caterpillars. Oecologia
Kukal O, Serianni AS, Duman JG (1988a) Glycerol metabolism in a freeze-tolerant arctic insect: an in vivo13C NMR study. J Comp Physiol B 158:175–183
Kukal O, Heinrich B, Duman JG (1988b) Behavioural thermoregulation in the freeze tolerant arctic caterpillar,Gynaephora groenlandica. J Exp Biol 138:181–193
Kurland CG, Schneidermann HA, Smith RD (1958) Oxygen debts in diapausing insects. Anat Rec 132:465–466
Lee AD (1955) The physiology of diapause in arthropods. Cambridge University Press, London
Lee RE, Chen C-P, Meacham MH, Denlinger DL (1987) Ontogenic patterns of cold-hardiness and glycerol production inSarcophaga crassipalpis. J Insect Physiol 33:587–592
Mansingh A, Smallman BN (1972) Variation in polyhydric alcohol in relation to diapause and coldhardiness in the larvae ofIsia isabella. J Insect Physiol 18:1565–1571
Mazur P (1984) Freezing of living cells: mechanisms and implications. Am J Physiol 247:125–142
Meyer SGE (1978) Effects of heat, cold, anaerobiosis and inhibitors on metabolite concentrations in larvae ofCallitroga macellaria. Insect Biochem 6:471–47
Meyer SGE (1980) Studies on anaerobic glucose and glutamate metabolism in larvae ofCallitroga macellaria. Insect Biochem 10:449–455
Minks AK (1967) Biochemical aspects of juvenile hormone action in the adultLocusta migratoria. Arch Neerl Zool 17:175
Nordin JH, Cui Z, Yin C (1984) Cold-induced glycerol accumulation byostrinia nubilalis larvae is developmentally regulated. J Insect Physiol 30:563–566
Plaxton W, Storey KB (1986) Glycolytic enzyme binding and metabolic control in anaerobiosis. J Comp Physiol B 156:635–640
Rojas RR, Lee RE, Luu T, Baust JG (1983) Temperature dependence-independence of antifreeze turnover inEurosta solidaginis (Fitch). J Insect Physiol 29:865–869
Salt RW (1958) Role of glycerol in producing abnormally low supercooling and freezing points in an insect,Bracon cephi (Gahan). Nature 181:1281
Schneiderman HA, Williams CM (1953) The physiology of insect diapause VII. The physiology of the Cecropia silkworm during diapause and during development. Biol Bull (Woods Hole, Mass) 105:320–334
Schneiderman HA, Williams CM (1954) Physiology of insect diapause. VIII. Qualitative changes in the metabolism of the Cecropia silkworm during diapause and development. Biol Bull (Woods Hole, Mass) 106:200–210
Scholander PF, Flagg W, Hoch RJ, Irving L (1963) Climatic adaptation in arctic and tropical poikilotherms. Physiol Zool 26:67–92
Scholander PF, Flagg W, Hoch RJ, Irving L (1954) Studies on the physiology of frozen plants and animals in the arctic. J Cell Comp Physiol 49:1–56
Selwyn MJ (1987) Holes in mitochondrial membranes. Nature 330:424–425
Shappirio DG, Williams CM (1957a) The cytochrome system of the Cecropia silkworms. I. Spectroscopic studies of individual tissues. Proc R Soc London B 147:218–232
Shappirio DG, Williams CM (1957b) The cytochrome system of the Cecropia silkworm II. Spectrophotometric studies of oxidative enzyme systems in the wing epithelium. Proc R Soc London B 147:233–246
Siegert KJ (1986) The effects of chilling and integumentary injury on carbohydrate and lipid metabolism in diapause and non-diapause pupae ofManduca sexta. Comp Biochem Physiol 85A:257–262
Siegert KJ (1987) Carbohydrate metabolism in starved first instar larvae ofMaduca sexta. Arch Insect Biochem Physiol 4:151–160
Siegert KJ, Ziegler R (1983) A hormone from the corpora cardiaca controls fat body glycogen phosphorylase during starvation in tobacco hornworm larvae. Nature 301:526–527
Somme L (1974) Anaerobiosis in some alpine Coleoptera. Norsk Ent Tidsskr 21:155–158
Storey KB, Storey JM (1983) Biochemistry of freeze tolerance in terrestrial insects. Trends Biochem Sci 8:242–245
Takehara I (1966) Natural occurrence of glycerol in the slug caterpillarMonema flavescens. Low Temp Sci B 14:1–34
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press. New York
Tsumuki H, Rojas RR, Storey KB, Baust JG (1986) The fate of [14C]glucose during cold-hardening inEurosta solidaginis (Fitch). Insect Biochem 17:347–352
Wilhelm RC, Schneiderman HA, Daniel LJ (1961) The effects of anaerobiosis on the giant silkwormsHyalophora cecropia andSamia cynthia with special reference to the accumulation of glycerol and lactic acid. J Insect Physiol 7:273–288
Williams JN Jr (1964) A method of the simultaneous quantitative estimation of cytochromes a, b, c1 and c in mitochondria. Arch Biochem Biophys 107:537–543
Williamson DH, Fennell DJ (1975) The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. In: Prescott DM (ed) Methods in cell biology, vol 12. Academic Press, pp 335–351
Wittekind D (1972) Fluorescence properties of the monoaminoacridines and some 2-aminoacridine derivatives. In: Thaer AA, Sernetz M (eds) Fluorescence techniques in biology. Springer, Berlin Heidelberg New York, pp 95–105
Wood FE, Nordin JH (1980) Activation of hexose monophosphate shunt during cold-induced glycerol accumulation byProtophormia terranovae. Insect Biochem 10:87–93
Wyatt GR (1960) Phosphorus compounds in insect development. Proc 4th Int Congr Biochem 12:161–178
Wyatt GR, Meyer WL (1959) The chemistry of insect hemolymph III. Glycerol. J Gen Physiol 42:1005–1011
Wyatt GR, Kalf GF (1958) Organic components of insect hemolymph. Proc 10th Int Congr Ent 2:333
Yancey PH, Clark ME, Hank SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222
Yi S, Yin C, Nordin JH (1987) The chilling-induced biosynthesis and secretion of glycerol byOstrinia nubilalis larval fat bodies in vitro. J Insect Physiol 33:523–528
Zachariassen KE (1985) Physiology of cold tolerance in insects. Physiol Rev 65:799–832
Zebe EC, McShan WH (1957) Lactic and α-glycerophosphate dehydrogenases in insects. J Gen Physiol 40:779–790
Ziegler R, Wyatt GR (1975) Phosphorylase and glycerol production activated by cold in diapausing silkworm pupae. Nature 254:622–625
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kukal, O., Duman, J.G. & Serianni, A.S. Cold-induced mitochondrial degradation and cryoprotectant synthesis in freeze-tolerant arctic caterpillars. J Comp Physiol B 158, 661–671 (1989). https://doi.org/10.1007/BF00693004
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00693004