Abstract
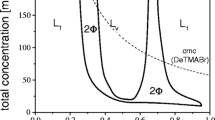
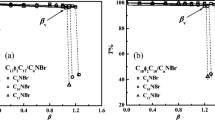
In the aqueous mixtures of sodium alkylcarboxylate and alkyltrimethylammonium bromide, large unilamelar vesicles can be formed spontaneously or by sonication as the total carbon number in the HC chains is 19 (or larger). Vesicle formation can be influenced by changes of pH, molar ratio of the two surfactant components, and the polar head group of cationic surfactant. Micelles may coexist with the vesicles in these mixed systems. The larger hydrodynamic radius (∼200 nm) and aggregation number (∼800) illustrate that the shape of the micelle in 1:1 C9H19COONa−C10H21N(CH3)3Br is rod-like. In some mixed systems, the micelles can be transformed into stable vesicles by sonication — a phenomenon revealed for the first time. The surface-chemical properties of these catanionic surfactant solutions and the stabilities of vesicle have been studied systematically.
Similar content being viewed by others
References
Fendler JH (1982) Membrane Mimetic Chemistry. Wiley interscince, New York, Ch 6
Kunitake T (1986) In: Mittal KL, Bothorel P (eds) Surfactants in Solution, Vol 5. Plenum, New York, 727
Israelachvili JN (1985) In: Intermolecular and Surface Force. Academic Press, London, Ch 16
Hua XY, Zhao GX, (1964) Acta Chim Sinica, 3:441
Zhao GX, Ou JG, Tian BS, Huang ZM (1980) Acta Chim Sinica, 38:409
Zhao GX, Zhu BY (1986) In: Scamehorn JF Eds. Phenomena in Mixed Surfactnat Systems, ACS Symp Sci 311, ACS, Washington D.C., 184
Yang WS, Zhao GX (1985) Acta Chim Sinica, 43:705
Yu ZJ, ZHao GX (1989) J Colloid Interface Sci, 130:141; 432
Li XG, Zhao GX (1992) Colloids Surf, 64:185
Kaler EW, Murthy AK, Rodriguez BE, Zasadzinski JAN (1989) Science, 245:1371
Zhao GX, Huang JB (1992) Acta Physico-chim. Sinica, 8:583
Zhu BY, Zhao GX (1981) HuaXueTongBao, 6:341
Zhao GX (1984) Physical Chemistry of Surfactant (Chinese). Peking University Press, Beijing, Ch. 3
Zana R (Eds) (1987) Surfactanta in Solutions: New methords of Investigation. Marcel Dekker Inc, New York, Ch. 5
Brown RF (1975) Organic Chemistry. Wadsworth, Belmont, Calif
Zhao GX, Huang JB (1992) China Surfactant Detergent and Cosmetics 5:7
Tanford C (1980) The Hydrophobic Effect (2nd Ed.) Wiley, New York
Israelachivili JN, Mitchell DJ, Ninham BW (1976) J Chem Soc, Faraday Trans II, 72:1525
Zhao GX (1984) Physical Chemistry of Surfactant (Chinese) Peking University Press, Beijing, Ch. 5
Zaslavsky BY, Borovskaya A, Lavrinenko AK, Lisichkin AY, Davidovich YA and Rogozhin SV (1980) Chem Phys Lett, 26:49
Kano K and Fendler JH (1979) Chem Phys Lipids, 23:189
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huang, J.B., Zhao, G.X. Formation and coexistence of the micelles and vesicles in mixed solution of cationic and anionic surfactant. Colloid Polym Sci 273, 156–164 (1995). https://doi.org/10.1007/BF00654013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00654013