Abstract
Isolated Langendorff perfused rat hearts were used to study changes in the Ca, Na and K content, contractile force and the loss of cellular material during the Ca paradox.
-
1.
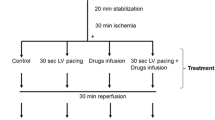
Five minutes perfusion with Ca-free solution containing 1 mM EGTA, followed by 10 min of reperfusion in 1.8 mM Ca causes irreversible contracture, K loss, increase in Na and Ca and a massive release of myoglobin and other cellular material into the perfusate (the calcium paradox).
-
2.
During the Ca-free perfusion the ventricles gain Na but the K content decreases slightly. The size of the Na gain appears to depend upon the buffer used and is larger in bicarbonate than in Tris.
-
3.
When HCO −3 or H2PO −4 ions are omitted from the bathing solution (in Tris, HEPES, or TES buffered salines) the adverse effects of Ca readmission are reduced. Tris buffer gives the best protection.
-
4.
Metabolic inhibition with FCCP (5×10−7 M), or with CN−(2×10−3 M) together with iodoacetic acid (2×10−3 M), decreases Ca uptake during the Ca paradox and inhibits the release of cellular material. In both cases a contracture is observed.
-
5.
Ruthenium red (10−4 M) does not inhibit the Ca readmission contracture but reduces the release of cellular material and the gain of Ca and Na.
The results suggest that the loss of cellular constituents during the calcium paradox, is related to an active uptake of Ca by the mitochondria and may lead to massive changes in the cellular ion concentration, during Ca-repletion.
Similar content being viewed by others
References
Alto LE, Dhalla NS (1979) Myocardial cation contents during induction of calcium paradox. Am J Physiol 237:H713-H719
Alto LE, Dhalla NS (1981) Role of changes in microsomal calcium uptake in the effect of reperfusion of Ca2+-deprived rat hearts. Circ Res 48:17–24
Baker JE, Bullock GR, Hearse DJ (1983) The temperature dependence of the calcium paradox: enzymatic, functional and morphological correlates of cellular injury. J Mol Cell Cardiol 15:393–411
Busselen P (1982) Effect of potassium depolarization on sodium-dependent calcium efflux from goldfish heart ventricles and guinea-pig atria. J Physiol 327:309–324
Bygrave FL (1978) Mitochondria and the control of intracellular calcium. Biol Rev 53:43–79
Chapman RA (1979) Excitation-contraction coupling in cardiac muscle. Prog Biophys Molec Biol 35:1–52
Chapman RA (1983) Control of cardiac contractility at the cellular level. Am J Physiol 245:H535-H552
Chapman RA, Rodrigo GC, Tunstall J, Yates RJ, Busselen P (1984) Intracellular sodium and the calcium paradox. Am J Physiol 247:H874-H879
Chesnais J-M, Corabocuf E, Sauviat M-P, Vassas J-M (1971) Inhibition par les ions magnésium de la conductance sodique lente apparaissant sous l'effet de dépolarisations membranaires au niveau des fibres atriales de Grenouille. CR Acad Sc Paris 273:1594–1597
Elder JA, Lehninger AL (1973) Respiration-dependent transport of carbon dioxide into rat liver mitochondria. Biochemistry 12:976–982
Forbes MS, Sperelakis N (1979) Ruthenium-Red staining of skeletal and cardiac muscles. Cell Tissue Res 200:367–382
Ganote CE (1983) Contraction band necrosis and irreversible myocardial injury. J Mol Cell Cardiol 15:67–73
Ganote CE, Grinwald P, Nayler WG (1983) 2,4-Dinitrophenol (DNP)-induced injury in calcium-free hearts. J Mol Cell Cardiol 15 (suppl 1) no 393
Garnier D, Rougier O, Gargouïl YM, Coraboeuf E (1969) Analyse éléctrophysiologique du plateau des réponses myocardiques, mise en évidence d'un courant lent entrant en absence d'ions bivalents. Pflügers Arch 313:321–342
Grinwald PM, Nayler WG (1981) Calcium entry in the calcium paradox. J Mol Cell Cardiol 13:867–880
Harris EJ (1978) Anion/Calcium ion ratios and proton production in some mitochondrial calcium ion uptakes. Biochem J 176: 983–991
Hearse DJ, Humphrey SM, Bullock GR (1978) The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol 10:641–668
Hearse DJ, Humphrey SM, Chain EB (1973) Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol 5:395–407
Holland CE Jr, Olson RE (1975) Prevention by hypothermia of paradoxical calcium necrosis in cardiac muscle. J Mol Cell Cardiol 7:917–928
Koomen JM, Noordhoek J, Zimmerman ANE (1983) Ca2+-free perfusion of isolated rat heart: early irreversible changes and discrepancy between functional impairments and release of cellular constituents. Cardiovascular Research 17:476–481
Lamers JMJ, Stinis JT, Ruigrok TJC (1984) Biochemical properties of membranes isolated from calcium-depleted rabbit hearts. Circ Res 54:217–226
Lehninger AL (1974) Role of phosphate and other proton-donating anions in respiration-coupled transport of Ca2+ by mitochondria. Proc Natl Acad Sci USA 71:1520–1524
Moore CL (1971) Specific inhibition of mitochondrial Ca2+ transport by Ruthenium Red. Biochem Biophys Res Commun 42:298–305
Muir AR (1968) A calcium-induced contracture of cardiac muscle cells. J Anat 102:148–149
Nahas GG (1962) The pharmacology of tris (hydroxymethyl)-aminomethan (THAM). Pharmac Rev 14:447–472
Nakanishi T, Nishioka K, Jarmakani JM (1982) Mechanism of tissue Ca2+ gain during reoxygenation after hypoxia in rabbit myocardium. Am J Physiol 242:H437-H449
Nayler WG, Grinwald PM (1982) Dissociation of Ca2+ accumulation from protein release in the calcium paradox: effect of barium. Am J Physiol 242:H203-H210
Nayler WG, Perry S, Daly MJ (1983) Cobalt, manganese and the calcium paradox. J Mol Cell Cardiol 15:735–747
Ogunro EA, Ferguson AG, Lesch MA (1980) A kinetic study of the pH optimum of canine cardiac cathepsin D. Cardiovasc Res 14:254–260
Poole-Wilson PA, Harding DP, Bourdillon PDV, Tones MA (1984) Calcium out of control. J Mol Cell Cardiol 16:175–187
Reuter H, Scholz H (1977) A study of the ion selectivity and the kinetic properties of the calcium-dependent slow inward current in mammalian cardiac muscle. J Physiol 264:17–47
Rich TL, Langer GA (1982) Calcium depletion in rabbit myocardium. Calcium paradox protection by hypothermia and cation substitution. Circ Res 51:131–141
Ruigrok TJC, Burgensdijk FJA, Zimmerman ANE (1975) The calcium paradox: a reaffirmation. Eur J Cardiol 3:59–63
Ruigrok TJC, Boink ABTJ, Spies F, Blok FJH, Maas AHJ, Zimmerman ANE (1978) Energy dependence of the calcium paradox. J Moll Cell Cardiol 10:991–1002
Somlyo AP, (1984) Cellular site of calcium regulation. Nature 309:516–517
Weglicki WB, Waite BM, Stam AC (1972) Association of phospholipase A with a myocardial membrane preparation containing the (Na++K+)-Mg2+-ATPase. J Mol Cell Cardiol 4:195–201
Zimmerman ANE, Hülsmann WC (1966) Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature 211:646–647
Zimmerman ANE, Daems W, Hülsmann WC, Snijder J, Wisse E, Durrer D (1967) Morphological changes of heart muscle caused by successive perfusion with calcium-free and calcium-containing solutions (calcium paradox). Cardiovasc Res 1:201–209
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Busselen, P. Suppression of cellular injury during the calcium paradox in rat heart by factors which reduce calcium uptake by mitochondria. Pflugers Arch. 404, 166–171 (1985). https://doi.org/10.1007/BF00585414
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00585414