Abstract
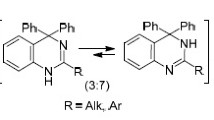
A method was developed for the preparation of 2,3-polymethylene-3,4-dihydro-4-quinazolones with substituents in the benzene and polymethylene chains by reaction of anthranilic acid and its substituted derivatives with γ-butyro-, 5-phenyl-γ-butyro-, ɛ-capro-, and α-chloro-ɛ-caprolactams in the presence of dehydrating agents. The corresponding quinazolines were obtained by reduction of the synthesized quinazolones.
Similar content being viewed by others
Literature cited
A. Irisbaev, Kh. M. Shakhidoyatov, and Ch. Sh. Kadyrov, Zh. Prirodn. Soedin., No. 4, 531 (1975).
S. Yu. Yunusov, Alkaloids [in Russian], Tashkent (1974), p. 169.
Kh. N. Khashamov, M. V. Telezhenetskaya, and S. Yu. Yunusov, Khim. Prirodn. Soedin., 456 (1969).
A. Chatterjee and M. Gonguly, Phytochem., 307 (1968).
R. S. Moris, W. F. Handford, and R. Adams, J. Amer. Chem. Soc., 57, 951 (1935).
E. Spath, F. Kuffner, and N. Platzer, Ber., 68, 2221 (1935).
S. Peterson and E. Tietze, US Patent No. 2992221 (1961).
H. Mohrle and C. M. Sildel, Ber., 106, 1595 (1973).
W. F. Handford and R. Adams, J. Amer. Chem. Soc., 57, 92 (1935).
E. Spath, F. Kuffner, and N. Platzer, Ber., 69, 255 (1936).
G. G. Muor and R. Madronero, Ber., 95, 2182 (1962).
J. Timathy, Frederic D. B. Domolo, Helene Van Holven, B. Paul, and L. Bernard, J. Med. Chem., 16, 633 (1973).
N. I. Koretskaya and A. M. Utkin, Zh. Obshch. Khim., 28, 1087 (1958).
A. Ettenne and Y. Correia, Bull. Soc. Chim. France, 3704 (1969).
H. Mohrle and P. Gandlach, Tetrahedron Lett., 3249 (1970).
Preparative Organic Chemistry [translated into Russian from Polish], Khimiya (1964), p. 509.
F. Ullmann and J. B. Uzbachian, Ber., 36, 1802 (1903).
A. S. Wheeler, J. Amer. Chem. Soc., 31, 566 (1909).
O. Yu. Magidson and E. S. Golovchinskaya, Zh. Obshch. Khim., 8, 1797 (1938).
Organic Syntheses [Russian translation], Vol. 2 (1943).
R. Tull, R. C. O. Nell, E. P. McCarthy, J. J. Papes, and M. J. Chemerda, J. Org. Chem., 29, 2425 (1964).
G. I. Nikishin, B. D. Vorob'ev, and A. D. Petrov, Dokl. Akad. Nauk SSSR, 136, 360 (1961).
Author information
Authors and Affiliations
Additional information
See [1] for communication V.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1564–1569, November, 1976.
Rights and permissions
About this article
Cite this article
Shakhidoyatov, K.M., Irisbaev, A., Yun, L.M. et al. Quinazolines. Chem Heterocycl Compd 12, 1286–1291 (1976). https://doi.org/10.1007/BF00470236
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00470236