Abstract
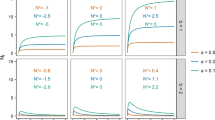
Models of complex life cycles predict that greater mortality of immature stages should induce earlier metamorphosis at smaller sizes. We tested for effects of one source of mortality, habitat drying, on size at and time to metamorphosis of the tree hole mosquito Aedes triseriatus. In a laboratory experiment, we manipulated two variables associated with drying, volume of water and solute concentration, and recorded mean mass at and median days to eclosion for males and females in replicate cohorts. We also tested for treatment effects on the correlation of mass at and time to eclosion. For females, decreasing volume consistently induced metamorphosis at smaller sizes than did constant volume. Decreasing volume also led to earlier metamorphosis of females than did constant volume, but only in one of two experimental runs. For both sexes, increasing concentration led to greater size at metamorphosis and, for males, earlier metamorphosis than did constant volume, but again only in one of two experimental runs. Correlations of size at and time to metamorphosis tended to be positive for females and negative for males, and this difference was significant. For both sexes, decreasing volume led to larger (more positive) correlations than did constant volume, but only in one of two experimental runs. The effects of decreasing volume on females are consistent with the predictions of models of complex life cycles, and suggest that A. triseriatus can perceive volume changes and modify metamorphosis to escape a deteriorating habitat. The effects of increasing concentration are opposite to those predicted, and are consistent with enhanced growth rates, possibly due to enhanced microbial growth, as solutes become concentrated due to drying. The responses of these mosquitoes to habitat drying are complex, and we suggest that habitat drying increases both mortality and growth rates, yielding no simple predictions of how habitat drying will affect these mosquitoes in natural tree holes.
Similar content being viewed by others
References
Alford RA, Harris RN (1988) Effects of larval growth history on anuran metamorphosis. Am Nat 131:91–106
Banks MJ, Thompson DJ (1987) Regulations of damselfly populations: the effects of larval density on survival, development rate and size in the field. Freshwater Biol 14:1–12
Bradshaw WE, Holzapfel CM (1988) Drought and the organization of tree-hole mosquito communities. Oecologia 74:507–514
Büns M, Ratte HT (1991) The combined effects of temperature and food consumption on body weight, egg production, and developmental time in Chaoborus crystallinus DeGeer (Diptera:Chaoboridae). Some new evidence for the adaptive value of vertical migration. Oecologia 88:470–476
Carpenter SR (1983) Resource limitation of larval treehole mosquitoes subsisting on beech detritus. Ecology 64:219–223
Chambers RC (1985) Competition and predation among larvae of three species of treehole breeding mosquitoes. In: Lounibos LP, Rey JR, Frank JH (eds) Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory, Vero Beach, pp 25–54
Chodorowski A (1969) The desiccation of ephemeral pools and the rate of development of Aedes communis larvae. Pol Arch Hydrobiol 16:79–91
Crump ML (1989) Effect of habitat drying on developmental time and size at metamorphosis. Copeia 1989:794–797
Fauth JE (1990) Interactive effects of predators and early larval dynamics of the treefrog Hyla chrysoscelis. Ecology 71:1609–1616
Figiel CR Jr, Semlitsch RD (1990) Population variation in survival and metamorphosis of larval salamanders (Ambystoma maculatum) in the presence and absence of fish predation. Copeia 1990:818–826
Fish D (1985) An analysis of adult size variation within natural mosquito populations. In: Lounibos LP, Rey JR, Frank JH (eds) Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory, Vero Beach, pp 419–430
Fisher IJ, Bradshaw WE, Kammeyer C (1990) Fitness and its correlates assessed by intra- and interspecific interactions among tree-hole mosquitoes. J Anim Ecol 59:819–829
Hard JJ, Bradshaw WE, Malarkey DJ (1989) Resource- and density-dependent development in tree-hole mosquitoes. Oikos 54:137–144
Juliano SA (1989) Geographic variation in vulnerability to predation and starvation in larval treehole mosquitoes. Oikos 56:99–108
Lounibos LP (1985) Interactions influencing production of treehole mosquitoes in south Florida. In: Lounibos LP, Rey JR, Frank JH (eds) Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory, Vero Beach, pp 65–78
Lounibos LP, Nishimura N, Escher RL (1993) Fitness of a treehole mosquito: influences of food type and predation. Oikos 66:114–118
Ludwig D, Rowe L (1990) Life history strategies for energy gain and predator avoidance under time constraints. Am Nat 135:686–707
McKone MJ, Lively CM (1993) Statistical analysis of experiments conducted at multiple sites. Oikos 67:184–186
Newman RA (1988) Adaptive plasticity in development of Scaphiopus couchii tadpoles in desert ponds. Evolution 42:763–773
Newman RA (1989) Developmental plasticity of Scaphiopus couchii tadpoles in an unpredictable environment. Ecology 70:1775–1787
Norvak RJ, Shroyer DA (1978) Eggs of Aedes triseriatus and A. hendersoni: a method to stimulate optimal hatch. Mosq News 38:515–521
Peckarsky BL, Cowan CA (1991) Consequences of larval intraspecific competition to stonefly growth and fecundity. Oecologia 88:277–288
Petranka JN, Sih A (1987) Habitat duration, length of larval period, and the evolution of a complex life cycle of a salamander Ambystoma texanum. Evolution 41:1347–1356
Pfennig DW (1992) Polyphenism in spadefoot toad tadpoles as a locally adjusted evolutionarily stable strategy. Evolution 46:1408–1420
Pfennig DW, Mabrey A, Orange D (1991) Environmental causes of correlations between age and size at metamorphosis in Scaphiopus multiplicatus. Ecology 72:2240–2248
Rowe L, Ludwig D (1991) Size and timing of metamorphosis in complex life cycles: Time constraints and variation. Ecology 72:413–427
Rucda LM, Patel KJ, Axtell RC, Stinner RE (1990) Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera:Culicidae). J Med Entomol 27:892–898
Semlitsch RD (1987a) Paedomorphosis in Ambystoma talpoideum: effects of density, food and pond drying. Ecology 68:994–1002
Semlitsch RD (1987b) Relationship of pond drying to reproductive success of the salamander Ambystoma talpoideum. Copeia 1987:61–69
Semlitsch RD, Gibbons JW (1985) Phenotypic variation in metamorphosis and paedomorphosis in the salamander Ambystoma talpoideum. Ecology 66:1123–1130
Semlitsch RD, Wilbur HM (1988) Effects of pond drying time on metamorphosis and survival in the salamander Ambystoma talpoideum. Copeia 1988:978–983
Skelly DK (1992) Field evidence for a cost of behavioral antipredator response in a larval amphibian. Ecology 73:704–708
Skelly DK, Werner EE (1990) Behavioral and life historical responses of larval American toads to an odonate predator. Ecology 71:2313–2322
Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ (1991) Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology 72:1529–1546
Werner EE (1986) Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am Nat 128:319–341
Werner EE (1988) Size, scaling, and the evolution of complex life cycles. In: Ebenman B, Persson L (eds) Size-structured populations: ecology and evolution. Springer, Berlin, pp 60–81
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst 15:393–425
Wilbur HM (1987) Regulation of structure in complex systems: experimental temporary pond communities. Ecology 68:1437–1452
Wilbur HM,(1988) Interactions between growing predators and growing prey. In: Ebenman B, Persson L (eds) Size-structured populations: ecology and evolution. Springer, Berlin, pp 157–172
Wilbur HM, Collins JP (1973) Ecological aspects of amphibian metamorphosis. Science 182:1305–1314
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Juliano, S.A., Stoffregen, T.L. Effects of habitat drying on size at and time to metamorphosis in the tree hole mosquito Aedes triseriatus . Oecologia 97, 369–376 (1994). https://doi.org/10.1007/BF00317327
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317327