Summary
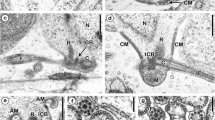
The mesogastropod Pyrazus ebeninus, produces true spermatozoa (here termed euspermatozoa) and multi-flagellate, mobile cells (here termed paraspermatozoa). The mature paraspermatozoon consists of an elongateconical ‘head’ (6.5–8.5 μm in length), constructed of an electron-dense mosaic sheath surrounding a similarly dense, rod-shaped nuclear core (which runs almost the full length of the head). An acrosome-like structure forms the apex of the head. Five to eight axonemes are fixed to the posterior extremity of the nuclear core, each by means of an attachment complex (dense attachment rod, centriolar cap and centriole). A short (3–4 μm) ‘midpiece’ zone follows the head and consists of the multiple axonemes interspersed with very elongate mitochondria. A tuft of short (20 μm) tails (termed minor tails) emerges from the midpiece in addition to one very long tail (termed the major tail) ensheathed in dense granules which resemble glycogen granules. A single membrane surrounds head, midpiece and tails whilst the nuclear core retains the original double nuclear membrane.
Developmentally, the multiple axonemes arise from one of a pair of wheel-shaped arrangements of centrioles and attach to posterior indentations in the nucleus prior to its transformation into the nuclear core. Dense vesicles, derived apparently from the endoplasmic reticulum, accumulate along and around the developing nuclear core and (in the presence of microtubules) condense into the mosaic head sheath. Cytoplasmic mitochondria elongate and collect at the posterior axis of the cell, where, together with the axonemes, they form the midpiece.
Features not previously reported in other ultrastructural studies of paraspermatozoa include the acrosome-like structure of the head, the structure of the midpiece zone, the glycogen sheath of the major tail, the dense annular structure at the junction of the midpiece and major tail and the presence of microtubules in the final phase of head and midpiece maturation. Some features of the euspermatozoon are also described and the comparative ultrastructure of mature and developing paraspermatozoa and their possible functions in the Gastropoda, are reviewed.
Similar content being viewed by others
Abbreviations
- ac :
-
euspermatozoon acrosomal cone
- ar :
-
euspermatozoon axial rod
- ax :
-
axoneme
- b :
-
dense block of mosaic sheath
- c :
-
centriole
- cc :
-
centriolar cap
- co :
-
cone of acrosome-like structure
- dr :
-
dense attachment rod
- dv :
-
dense vesicle
- g :
-
glycogen granules
- G :
-
Golgi complex
- GER :
-
granular endoplasmic reticulum
- H :
-
head of paraspermatozoon
- m :
-
mitochondrion
- M :
-
midpiece (euspermatozoon, paraspermatozoon)
- maj :
-
major tail
- min :
-
minor tails
- mt :
-
microtubules
- n :
-
nucleus
- nc :
-
nuclear core
- p :
-
dense plug of acrosome-like structure
- pm :
-
plasma membrane
- sGv :
-
small Golgi vesicles
- Z :
-
transition of centriole to centriolar cap of attachment complex
References
Anderson WA, Personne P (1970) The localization of glycogen in spermatozoa of various invertebrate and vertebrate species. J Cell Biol 44:29–51
Anderson WA, Personne P (1976) The molluscan spermatozoon: dynamic aspects of its structure and function. Am Zool 16:293–313
Ankel WE (1926) Spermiozeugmenbildung durch atypische (apyrene) und typische Spermien bei Scala und Janthina. Verh Dtsch Zool Ges 31:193–202
Ankel WE (1930) Die atypische Spermatogenese von Janthina (Prosobranchia, Ptenoglossa). Z Zell-forsch Mikrosk Anat 11:491–608
Baccetti B, Afzelius BA (1976) The biology of the sperm cell. S. Karger Basel
Brock J (1987) Über die doppelten Spermatozoen einiger exotischer Prosobranchier. Zool Jahrb 2:615–624
Brunn M von, Untersuchungen über die doppelte Form der Samenkörper von Paludina vivipara. Arch Mikrosk Anat Entwicklungsmech 23:413–499
Buckland-Nicks JA (1973) The fine structure of the spermatozoon of Littorina with special reference to sperm motility. Z Zellforsch Mikrosk Anat 144:11–29
Buckland-Nicks JA, Chia FS (1977) On the nurse cell and the spermatozeugma in Littorina sitkana. Cell Tissue Res 179:347–356
Bulnheim HP (1962) Elektronenmikroskopische Untersuchungen zur Feinstruktur der atypischen und typischen Spermatozoen von Opalia crenimarginata (Gastropoda, Prosobranchia). Z Zell-forsch Mikrosk Anat 56:371–386
Bulnheim HP (1968) Atypische Spermatozoenbildung bei Epitonium tinctum. Ein Beitrag zum Problem des Spermatozoendimorphismus der Prosobranchia. Holgol wiss Meeresunters 18:232–253
Dallai R (1979) An overview of atypical spermatozoa in insects. In: Fawcett DW, Bedford JM (eds) The spermatozoon, Urban & Schwarzenberg, Baltimore Munich pp 253–265
Dembski WJ (1968) Histochemische Untersuchungen über Funktion und Verbleiben eu- und oligopyrener Spermien von Viviparus contectus (Millet, 1813), (Gastropoda, Prosobranchia). Z Zell-forsch Mikrosk Anat 89:151–179
Dixon BC (1915) Tubifex Liverpool Marine Biology Committee. Memoirs on typical British marine plants and animals. Herdmann WA (ed). (XXIII, 1–100)
Dupouy J (1960) Phénomènes dégénératifs, spermatozoïdes atypiques et oocytes atypiques chez un opisthobranche Haminea navicula Da Costa. Cellule 61:99–106
Dupouy J, Tuzet O, Galangau V (1966) Ultrastructure de la spermatide atypique dans la lignée apyrène de Columbella rustica L. (Gastéropode, Prosobranche). CR Hebd Seanc Acad Sci Paris 262:2251–2254
Fadda G (1924) Origine, sviluppo et funzione degli pseudospermatozoi di Murex trunculus L. Memorie R Comm Talassogr Ital 112:1–16
Fain-Maurel MA (1966) Acquisitions récentes sur les spermatogenèses atypiques Ann Biol 5:513–564
Fawcett DW, Anderson WA, Phillips DM (1971) Morphogenetic factors influencing the shape of the sperm head. Devl Biol 26:220–251
Folliot R (1979) Ultrastructural study of spermiogenesis of the anuran amphibian Bombina variegata. In: Fawcett DW, Bedford JM (eds) The spermatozoon. Urban & Schwarzenberg, Baltimore-Munich pp 333–339
Franzén Å (1956) On spermiogenesis, morphology of the spermatozoon, and the biology of fertilization among invertebrates. Zool Bidr Upps 31:356–482
Fretter V, Graham A (1962) British prosobranch molluscs. Their functional anatomy and ecology. Ray Society London
Gall JG (1961) Centriole replication. A study of spermatogenesis in the snail Viviparus. J Biophys Biochem Cytol 10:163–193
Gatenby JB (1919) The cytoplasmic inclusions of the germ-cells. IV. Notes on the dimorphic spermatozoa of Paludina and the giant germ-nurse cells of Testacella and Helix. Q J Micros Sci 63:401–443
Goldschmidt R (1916) The function of the apyrene spermatozoa. Science 44:544–546
Graham A (1954) Some observations on the reproductive tract of Janthina janthina (L.) Proc Malacol Soc London 31:1–6
Gutherz S (1926) Zur kausalen Analyse des Spermien Dimorphismus. Biol Zbl 46:508–527
Hachiri S, Higashi S (1972) Utilization of glycogen in spermatozoa of pond snails Sinotaia histrica and Heterogen longispira. Mem Fac Educ Shiga Univ Nat Sci 22:43–57
Hadfield MG (1969) Nurse eggs and giant sperm in the Vermetidae. Am Zool 9:1141–1142
Hanson J, Randall JT, Bayley ST (1952) The microstructure of the spermatozoa of the snail Viviparus. Exp Cell Res 3:65–78
Hertwig, R (1905) Über das Problem der sexuellen Differenzierung. Verh Dtsch Zool Ges 15:186–214
Hyman OW (1925) Natural partial fertilization in Fasciolaria tulipa. J Morphol 41:267–281
Ishizaki T, Kato K (1958) The fine structure of atypical spermatozoa of the pond snail Viviparus malleatus. Zool Mag Tokyo 286–294
Jamieson BGM, Daddow L (1979) An ultrastructural study of microtubules and the acrosome in spermiogenesis of Tubificidae (Oligochaeta). J Ultrastruct Res 67:209–224
Kuschakewitsch S (1910) Zur Kenntnis der sogenannten „wurmförmigen” Spermien der Prosobranchier. Anat Anz 37:318–324
Kuschakewitsch S (1921) Studien über den Dimorphismus der männlichen Geschlechtselemente bei den Prosobranchia II. Die Spermatogenese von Cerithium vulgatum L. Arch Zellforsch 15:313–369
Lucas A (1971) Les gametes des mollusques. Haliotis 1:185–214
Mattei X (1970) Spermiogenese comparee des poissons. In: Baccetti B (ed). Comparative spermatology Academic Press, New York, pp 57–69
Mehra HR (1926) Cytoplasmic organs in the germ cells and somatic cells of Tubifex. Allahabad University Studies 3:1–56
Melone G, Lora Lamia Donin C, Cotelli F (1978) Aspetti ultrastrutturali degli spermatozoi atipici in Scalidae (Gastropoda, Prosobranchia). Boll Zool 45:261–268
Melone G, Lora Lamia Donin C, Cotelli F (1980) The paraspermatic cell (atypical spermatozoon) of Prosobranchia: a comparative ultrastructural study. Acta Zool (Stockholm) 61:191–201
Meves F (1903) Über oligopyrene und pyrene Spermien und über ihre Entstehung nach Beobachtungen an Paludina und Pygaera. Arch Mikrosk Anat 61:1–84
Neuhaus W (1959) Die Resorption eu- und dyspyrener Spermien bei Bithynia tentaculata. Zool Anz 163:160–167
Nickerson SC, Smith JJ, Keenan TW (1980) Role of microtubules in milk secretion — action of colchicine on microtubules and exocytosis of secretory vesicles in rat mammary epithelial cells. Cell Tissue Res 207:361–376
Nishiwaki S (1964) Phylogenetical study on the type of the dimorphic spermatozoa in Prosobranchia. Sci Rep Tokyo Kyoiku Daig 2:237–275
Pitelka DR (1969) Centriole replication. In: Lima-De-Faria A (ed) Handbook of molecular cytology. North Holland Publishing Co., Amsterdam, pp 1199–1218
Popoff M (1907) Eibildung bei Paludina vivipara und Chromidien bei Paludina und Helix. Arch Mikrosk Anat 70:43–129
Reinke EE (1911) Report upon the dimorphic spermatozoa of marine prosobranchs. Carnegie Inst Washington Yearb 10:133–136
Reinke EE (1912) A preliminary account of the development of the apyrene spermatozoa in Strombus and of the nurse cells in Littorina. Biol Bull Mar Biol Lab Woods Hole 22:319–327
Reinke EE (1914) The development of the apyrene spermatozoa of Strombus bituberculatus. Pap Tortugas Lab 183:195–239
Rosati F, Baccetti B, Dallai R (1970) The spermatozoon of Arthropoda. X Araneids and the lowest myriopods. In: Baccetti B (ed) Comparative spermatology. Academic Press, New York pp 247–254
Sharma GP, Gupta BL, Mittal OP (1959) Spermatogenesis of the gastropod Pila globosa with special reference to the cytoplasmic organelles. Cytologia 24:423–437
Stephan P (1903) Le développement des spermies apyrènes de Cerithium vulgatum et de Nassa mutabilis. Biblphie Anat 12:77–82
Tanaka H (1958) Electron microscopic studies on the sperm dimorphism. Acta Anat Nippon 33:387–410
Thompson TE (1973) Euthyneuran and other molluscan spermatozoa. Malacologia 14:167–206
Tochimoto T (1967) Comparative histochemical study on the dimorphic spermatozoa of the Prosobranchia with special reference to polysaccharides. Sci Rep Tokyo Kyoiku Daig 13:75–109
Tuzet O (1930) Recherches sur la spermatogenèse des prosobranches. Archs Zool Exp Gen 70:95–229
Werner G (1970) On the development and structure of the neck in urodele sperm. In: Baccetti B (ed) Comparative spermatology Academic Press, New York, pp 85–91
Woodard TM Jr (1940) The function of the apyrene spermatozoa of Goniobasis laqueata (Say). 1 The behaviour of the apyrene and eupyrene spermatozoa under natural and artificial conditions. J Exp Zool 85:103–125
Yasuzumi G (1962) Spermatogenesis in animals as revealed by electron microscopy XII. Light and electron microscope studies on spermiogenesis of Cipangopaludina malleata Reeve. J Ultrastruct Res 7:488–503
Yasuzumi G (1964) Spermatogenesis in animals as revealed by electron microscopy X. The fine structure and function of endoplasmic reticulum and of peculiar bodies appearing in atypical maturing spermatids and nutritive cells of Cipangopaludina malleata Reeve. Am J Anat 115:431–472
Yasuzumi G, Tanaka H (1958) Spermatogenesis in animals as revealed by electron microscopy. VI. Researches on the spermatozoon-dimorphism in a pond snail Cipangopaludina malleata. J Biophys Biochem Cytol 4:621–632
Yasuzumi G, Ishida H, Nakano S, Yamamoto H (1960) Spermatogénèse des animaux révélée par le microscope électronique. IX. Etude au microscope électronique de la télophase des spermatocytes de Cipangopaludina malleata et de Gelastorrhinus bicoler de Haan, avec des remarques sur les Nebenkerns. J Ultrastruct Res 3:484–494
Yasuzumi G, Nakano S, Matsuzaki W (1962) Elektronenmikroskopische Untersuchungen über die Spermatogenese. XI. Über die Spermiogenese der atypischen Spermatiden von Melania libertina Gould. Z Zellforsch Mikrosk Anat 57:495–511
Yasuzumi G, Lee KJ, Fukui H, Yoshida M (1967) Spermatogensis in animals as revealed by electron microscopy. XVII. The fine structure of atypical spermatid cytoplasm of the pond snail with particular reference to the site of hydraulic breakdown of nucleic acids. Z Zellforsch Mikrosk Anat 80:353–369
Yasuzumi G, Tsubo I, Matsuzaki W (1970a) Electron microscope observations on the chemical component of the sheath of the middle piece of atypical spermatozoa of the pond snail Cipangopaludina malleata Reeve. Arch Histol Jap 31:283–289
Yasuzumi G, Tsubo I, Yasuda M, Sugioka T, Sakamoto H, Yasuzumi F (1970b) Electron microscope studies on atypical spermatids of pond snail Cipangopaludina malleata Reeve, under consideration of conversion of DNA into Polysaccharide. In: Baccetti B (ed) Comparative spermatology, Academic Press, New York pp 401–413
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Healy, J.M., Jamieson, B.G.M. An ultrastructural examination of developing and mature paraspermatozoa in Pyrazus ebeninus (Mollusca, Gastropoda, Potamididae). Zoomorphology 98, 101–119 (1981). https://doi.org/10.1007/BF00310431
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00310431