Abstract
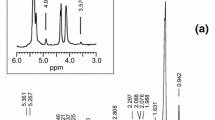
Species of the genus Lesquerella, within the Brassicaceae family, have seed oils containing hydroxy fatty acids. In most Lesquerella species, either lesquerolic (14-hydroxy-eicosa-11-enoic), auricolic (14-hydroxy-eicosa-11,17-dienoic) or densipolic (12-hydroxy-octadeca-9,15-dienoic) acid dominates in the seed oils. Incubations of developing seed from Lesquerella species with 1-14C-fatty acids were conducted in order to study the biosynthetic pathways of these hydroxylated fatty acids. [14C]Oleic (octadeca-9-enoic) acid, but not [14C]linoleic (octadeca-9,12-dienoic) acid, was converted into the hydroxy fatty acid, ricinoleic (12-hydroxy-octadeca-9-enoic) acid, which was rapidly desaturated to densipolic (12-hydroxy-octadeca-9,15-dienoic) acid. In addition, [14C] ricinoleic acid added to Lesquerella seeds was efficiently desaturated at the Δ15 carbon. A pathway for the biosynthesis of the various hydroxylated fatty acids in Lesquerella seeds is proposed. The demonstration of desaturation at position Δ15 of a fatty acid with a hydroxy group at position Δ12 in Lesquerella prompted a comparison of the substrate recognition of the desaturases from Lesquerella and linseed. It was demonstrated that developing linseed also was able to desaturate ricinoleate at position Δ15 into densipolic acid. In addition, the linseed Δ15 desaturase was able to desaturate vernolic (12,13-epoxy-octadeca-9-enoic) acid and safflower microsomal Δ12 desaturase was able to desaturate 9-hydroxy-stearate. Thus, hydroxy and epoxy groups may substitute for double bonds in substrate recognition for oil-seed Δ12 and Δ15 desaturases.
Similar content being viewed by others
Abbreviations
- GLC:
-
gas-liquid chromatography
- lysoPC:
-
palmitoyl-lysophosphatidylcholine
- PC:
-
phosphatidylcholine
References
Appelqvist L-Å (1972) A simple and convenient procedure for the hydrogenation of lipid on the micro and nanomole scale. J Lipid Res 13: 146–148
Badami RC, Patil KB (1981) Structure and occurrence of unusual fatty acids in minor seed oils. Prog Lipid Res 19: 119–153
Bafor M, Smith MA, Jonsson L, Stobart K, Stymne S (1991) Ricinoleic acid biosynthesis and triacylglycerol assembly in microsomal preparations from developing castor-bean (Ricinus communis) endosperm. Biochem J 280: 507–514
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917
Browse JP, Somerville CR (1991) Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Mol Biol 42: 467–506
Browse J, McCourt P, Somerville CR (1986) A mutant of Arabidopsis deficient in the C18∶3 and C16∶3 leaf lipids. Plant Physiol 81: 859–864
Browse J, Kunst L, Anderson S, Hughly S, Somerville C (1989) A mutant of Arabidopsis deficient in the chloroplast 16: 1/18: 1 desaturase. Plant Physiol 90: 522–529
Dutta PC, Appelqvist L-Å, Stymne S (1992) Utilization of petroselinate (C18: 1Δ6) by glycerol acylation enzymes in microsomal preparations of developing embryos of carrot (Daucus carola L.) safflower (Carthamus tinctorius L.) and oil rape (Brassica napus L.) Plant Sci 81: 57–64
Hayes DG, Kleiman R, Phillis BS (1995) The triacylglycerol composition, structure and presence of estolides in the oils of Lesquerella and related species. J Am Oil Chem Soc, in press
Hitz WD, Carlson TJ, Booth JR, Kinney AJ, Stecca KL, Yadav NS (1994) Cloning of a higher-plant plastid omega-6 fatty acid desaturase cDNA and its expression in cyanobacterium. Plant Physiol 105: 635–641
Howling D, Morris LJ, Gurr MI, James AT (1972) The specificities of fatty acid desaturases and hydroxylases. The dehydrogenation and hydroxylation of monoenic acids. Biochim Biophys Acta 260: 10–19
Miquel M, Browse J (1992) Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. J Biol Chem 267: 1502–1509
Morris LJ (1970) Mechanisms and stereochemistry in fatty acid metabolism. Biochem J 118: 681–693
Smith MA, Jonsson L, Stymne S, Stobart K (1992) Evidence for cytochrome b 5 as an electron donor in ricinoleic acid biosynthesis in microsomal preparations from developing castor bean (Ricinus communis L.). Biochem J 287: 141–144
Stahl U, Banas A, Stymne S (1995) Plant microsomal phospholipid acyl hydrolases have selectivities for uncommon fatty acids. Plant Physiol 107: 953–962
Stobart AK, Stymne S (1985) The regulation of the fatty acid composition in microsomal preparations from avocado mesocarp and the developing cotyledons of safflower. Planta 163: 119–125
Stobart AK, Stymne S, Höglund S (1986) Safflower microsomes catalyse oil accumulation in vitro: A model system. Planta 169: 33–37
Stymne S, Tonnet ML, Green AG (1992) Biosynthesis of linolenate in developing embryos and cell-free preparations of high-linolenate linseed (Linum usitatissimum) and low-linolenate mutants. Arch Biochem Biophys 284: 557–563
Author information
Authors and Affiliations
Additional information
This work was supported by grants from Stifteisen Svensk Oljeväxtforskning, Skanska Lantmännen Foundation, Swedish Farmers Foundation for Agricultural research, The Swedish Natural Science Research Council and The Swedish Council for Forestry and Agricultural Research. Nicki Engeseth was supported by the National Science Foundation under a grant award in 1992.
Rights and permissions
About this article
Cite this article
Engeseth, N., Stymne, S. Desaturation of oxygenated fatty acids in Lesquerella and other oil seeds. Planta 198, 238–245 (1996). https://doi.org/10.1007/BF00206249
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00206249