Abstract
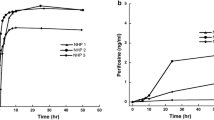
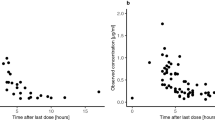
Mitoxantrone 5–6 mg/m2 was administered IV to 10 consenting patients prior to surgical resection of an intracerebral tumor. Plasma pharmacokinetic parameters were calculated and concentration of mitoxantrone in intracerebral tumors was determined. Concentrations of mitoxantrone were also determined in autopsy tissues of one of the patients who expired 192 days after receiving the drug. The plasma pharmacokinetics were best described by a 3 compartment model, with a t1/2γ of 4.74±5.53 h. Mitoxantrone concentrations in the intracerebral tumors were potentially cytotoxic and ranged from 4 to 322 ng/g. In all but one case, mitoxantrone concentration was higher in tumor than in concurrent plasma samples. There was no obvious relation between tumor mitoxantrone concentration and peak plasma mitoxantrone concentration or time from mitoxantrone administration to tumor removal. Low grade gliomas and viable tumors tended to have lower mitoxantrone concentrations than did other tumor types and necrotic tumors. In the patient undergoing autopsy, highest mitoxantrone concentrations were found in liver, thyroid and heart.
Similar content being viewed by others
References
White RJ, Durr FE: Development of mitoxantrone. Invest New Drugs 3:85–93, 1985
Prentice HG, Robbins G, Ma DDF, Ho AD: Sequential studies on the role of mitoxantrone in the treatment of acute leukemia. Cancer Treat Rep 10 (Suppl B):57–65, 1983
Gams RA, Kelley JW, Colomb HM, Steinberg J, Dukart G: Mitoxantrone in malignant lymphomas. Cancer Treat Rev 10 (Suppl B):69–72, 1983
Neidhart J, Goehnour D, Roach RW, Steinberg J, Young D: Mitoxantrone versus doxorubicin in advanced breast cancer: a randomized cross-over trial. Cancer Treat Rev 10 (Suppl B):41–46, 1983
Smith I: Mitoxantrone (novantrone): a review of experimental and early clinical studies. Cancer Treat Rev 10:103–115, 1983
Alberts DS, Peng YM, Bowden T, Dalton WS, Mackel C: Pharmacology of mitoxantrone: mode of action and pharmacokinetics. Invest New Drugs 3:101–107, 1985
Alberts DS, Peng Y-M, Leigh S, Davis TP, Woodward DL: Disposition of mitoxantrone in cancer patients. Cancer Res 45:1879–1884, 1985
Ehninger G, Proksch B, Heinzel G, Schiller E, Weible K-H, Woodward DL: The pharmacokinetics and metabolism of mitoxantrone in man. Invest New Drugs 3:109–116, 1985
Savaraj N, Lu K, Valdivieso M, Loo TL: Pharmacology of mitoxantrone in cancer patients. Cancer Chemother Pharmacol 8:113–117, 1982
Roboz J, Paciucci P, Silides D, Greaves J, Holland J: Detection and quantitation of mitoxantrone in human organs. A case report. Cancer Chemother Pharmacol 13:67–68, 1984
Stewart DJ, Leavens M, Maor M, Feun L, Luna M, Bonura J, Caprioli R, Loo TL, Benjamin RS: Human central nervous system distribution of cis-diamminedichloroplatinum and use as a radiosensitizer in malignant brain tumors. Cancer Research 42:2474–2479, 1982
Stewart DJ, Richard M, Hugenholtz H, Dennery J, Belanger R, Gerin-Lajoie J, Montpetit V, Nundy D, Prior J, Hopkins H: Penetration of VP-16 (etoposide) into human intracerebral and extracerebral tumors. J Neuro-Oncology 2:133–139, 1984
Stewart DJ, Lu K, Benjamin RS, Leavens M, Luna M, Yap HY, Loo TL: Concentrations of vinblastine in human intracerebral tumor and other tissues. J Neuro-Oncology 1:139–144, 1983
Stewart DJ, Benvenuto JA, Leavens M, Hall SW, Benjamin RS, Plunkett W, McCredie KD, Burgess MA, Loo TL: Penetration of 3-deazauridine into human brain, intracerebral tumor and cerebrospinal fluid. Cancer Research 39:4119–4122, 1977
Stewart DJ, Leavens M, Friedman J, Benjamin RS, Moore EC, Bodey GP, Valdivieso M, Burgess MA, Wiseman C, Loo TL: Penetration of N-(phosphonacetyl)-L-aspartate into human central nervous system and intracerebral tumor. Cancer Research 40:3163–3166, 1980
Rosenblum M, Stewart DJ, Yap BS, Leavens M, Benjamin RS, Loo TL: Penetration of methylglyoxal bis(guanylhydrazone) into intracerebral tumors in humans. Cancer Research 41:459–462, 1981
Savaraj N, Lu K, Feun LG, Leavens ME, Stewart D, Burgess MA, Benjamin RS, Loo TL: Intracerebral penetration and tissue distribution of 2,5-diaziridinyl 3,6-bis(carboethoxyamino)1,4-benzoquinone (AZQ, NSC-182986). J Neuro-Oncology 1:15–19, 1983
Zhengang G, Savaraj N, Feun LG, Lu K, Stewart DJ, Luna M, Benjamin RS, Loo TL: Tumor penetration of AMSA in man. Cancer Invest 1:475–478, 1983
Stewart DJ, Leavens M, Lu K, Wang YM, Benjamin RS, Ho DH, Yap HY, Loo TL: Central nervous system pharmacology of Baker's antifolate (NSC 139105) in man. J Neuro-Oncology 2:187–193, 1984
Stewart DJ, Richard MT, Hugenholtz H, Dennery J, Nundy D, Prior J, Montpetit V, Hopkins HS: Penetration of teniposide (VM-26) into human intracerebral tumors: preliminary observations on the effect of tumor type, rate of drug infusion and prior treatment with amphotericin-B or oral glycerol. J Neuro-Oncology 2:315–324, 1984
Stewart DJ, Benvenuto J, Leavens M, Smith R, Cabanillas F, Benjamin RS, Loo TL: Human central nervous system pharmacology of pentamethylmelamine and its metabolites. J Neuro-Oncology 1:357–364, 1983
Green RM, Hugenholtz H, Richard M, Dennery J, Hopkins H, Thibault M, Stewart DJ: Human central nervous system pharmacology of tiazofurin. Proceedings of the Second International Symposium on Biology of Brain Tumor. J Neuro-Oncology 2:288, 1984
Groothuis D, Fischer J, Vick N, Bigner D: Comparative permeability of different glioma models to horseradish peroxidase. Cancer Treat Rep 65 (Suppl 2):13–18, 1981
Stewart DJ, Hugenholtz H, Russell N, Richard MT, Benoit B, Maroun JA, Grahovac Z, Girard A, Nabwangu JF: Phase II Study of Novantrone (Mitoxantrone Hydrochloride) in Adults with Grade III–IV Astrocytomas. In: Walker MD, Thomas DGT (eds) Biology of Brain Tumor, Martinus Nijhoff Publishers, Boston, pp 411–413, 1986
Ostroy F, Gams RA: An HPLC method for the quantitative determination of 1,4-dihydroxy-5,8 bis [2-[(2-hydroxyethyl)-amino]ethyl]amino]9, 10-anthracenedione (DHAQ, Lederle Labs CL232 315, NCS 301739) in serum. J Liq Chromatog 3:637–644, 1980
Hulhoven R, Desager JP: HPLC determination of mitoxantrone in biological fluids: A sensitive and accurate method. J High Resolut Chromatog and Chromatog Commun 6:512–513, 1983
Sedman AJ, Wagner JG: CSTRIP, a Fortran IV Computer Program for obtaining initial polyexponential parameter estimates. J Pharm Sci 65:1006–1010, 1976
Loo JCK, Riegelman S: Assessment of pharmacokinetic constants from post infusion blood curves obtained after IV infusion. J Pharm Sci 59:53–55, 1970
Von Hoff DD, Coltman Jr CA, Forseth B: Activity of mitoxantrone in a human tumor cloning system. Cancer Res 41:1853–1855, 1981
Cowan JD, Von Hoff DD, Clark GM: Comparative cytotoxicity of adriamycin, mitoxantrone and bisantrene as measured by a human tumor cloning system. Invest New Drugs 1:139–144, 1983
Traganos F, Evenson DP, Staiano-Coico L, Darzynkiewicz Z, Melamed MR: Action of dihydroxyanthraquinone on cell cycle progression and survival of a variety of cultured mammalian cells. Cancer Res 40:671–681, 1980
Levin VA: A pharmacological basis for brain tumor chemotherapy. Sem Oncol 2:57–61, 1975
Broder LE, Rall DP: Chemotherapy of brain tumors. Prog Exp Tumor Res 17:373–399, 1972
Stewart DJ, Green RM, Mikhael NZ, Montpetit V, Thibault M, Maroun JA: Human autopsy tissue concentrations of mitoxantrone. Can Treat Rep 70:1255–1261, 1986
Bischoff K: Some fundamental considerations of the applications of pharmacokinetics to cancer chemotherapy. Cancer Chemother Rep 59:777–793, 1975
Posner LE, Dukart G, Goldberg J, Bernstein T, Cartwright K: Mitoxantrone: an overview of safety and toxicity. Invest New Drugs 3:123–132, 1985
Crossley R: Clinical safety and tolerance of mitoxantrone (novantrone). Cancer Treat Rev 10 (Suppl B):29–36, 1983
Stewart DJ, Hugenholtz H, Green R, Richard M, Benoit B, Russell N, Maroun J, Thibault M: Mitoxantrone hydrochloride: Uptake into human brain tumors and phase II study in gliomas. J Neuro-Oncology 4:114, 1986
Stewart DJ, Green R, Montpetit V, Hugenholtz H, Richard M, Mikhael N, Maroun J: Human tissue (tis) concentrations (conc) of mitoxantrone (m). Proc Amer Assoc Can Res 27:167, 1986
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Green, R.M., Stewart, D.J., Hugenholtz, H. et al. Human central nervous system and plasma pharmacology of mitoxantrone. J Neuro-Oncol 6, 75–83 (1988). https://doi.org/10.1007/BF00163544
Issue Date:
DOI: https://doi.org/10.1007/BF00163544