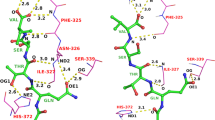
Summary
The program DOCK [1,2] has been used successfully to identify molecules which will bind to a specified receptor [3]. The original method ranks molecules based on their shape complementarity to the receptor site and relies on the chemist to bring the appropriate electrostatic or hydrogen bond properties into the molecular skeletons obtained in the search. This is useful when screening a small database of compounds, where it is not likely that molecules with both the correct shape and electrostatic properties will be found. As large databases are more likely to have redundant molecular shapes with a variety of functionality (e.g., members of a congeneric series), it would be useful to have a method which identifies molecules with both the correct shape and functionality. To this end we have modified the DOCK 1.0 method to target user-specified atom types to selected positions in the receptor site. The target sites can be chosen based on structural evidence, calculation or inspection. Targeted-DOCK improves the ability of the DOCK method to find the crystallographically determined binding mode of a ligand. Additionally, targeted-DOCK searches a database of small molecules at 100–1000 times the rate of DOCK 1.0, allowing more molecules to be screened and more sophisticated scoring schemes to be employed. Targeted-DOCK has been used successfully in the design of a novel non-peptide inhibitor of HIV-1 protease.
Similar content being viewed by others
References
Kuntz, I.D., Blaney, J.M., Oatley, S.J., Langridge, R. and Ferrin, T.E., J. Mol. Biol., 161 (1982) 269.
DesJarlais, R.L., Sheridan, R.P., Seibel, G.L., Dixon, J.S., Kuntz, I.D. and Venkataraghavan, R., J. Med. Chem., 31 (1988) 722.
Kuntz, I.D., Science, 257 (1992) 1078.
Moon, J.B. and Howe, W.J., Protein Struct. Funct. Genet., 11 (1991) 314.
Böhm, H.-J. J. Comput.-Aided Mol. Design, 6 (1992) 61.
Lewis, R.A., Roe, D.C., Huang, C., Ferrin, T.E., Langridge, R. and Kuntz, I.D., J. Mol. Graphics, 10 (1992) 66.
Meng, E.C., Shoichet, B.K. and Kuntz, I.D., J. Comput. Chem., 13 (1992) 505.
Leach, A.R. and Kuntz, I.D., J. Comput. Chem., 13 (1992) 730.
Chenera, B., DesJarlais, R.L., Finkelstein, J.P., Eggleston, D.S., Meek, T.D., TomaszekJr., T.A. and Dreyer, G.B., Bioorg. Med. Chem. Lett., 3 (1993) 2717.
Richards, F.M., Annu. Rev. Biophys. Bioeng. 6 (1977) 151.
Connoliy, M.I., Science, 221 (1983) 700.
Goodford, P.J., J. Med. Chem., 28 (1985) 849.
Miranker, A. and Karplus, M., Protein Struct. Funct. Genet., 11 (1991) 29.
Bernstein, F.C., Koetzle, T.F., Williams, G.J., Meyer, E.F., Brice, M.D., Rodgers, J.R. Kennard, O., Shimanouchi, T. and Tasumi, M., J. Mol. Biol., 112 (1977) 535.
Abola, E., Bernstein, F.C., Bryant, S.H., Koetzle, T.F. and Weng, J., In Allen, F.H., Bergerhoff, G. and Sievers, R. (Eds.) Crystallographic Database-Information Content, Software Systems, Scientific Applications, Data Commission of the International Union of Crystallography, Cambridge, 1987, p. 107.
Weis, W.I., Bruenger, A.T., Skehel, J.J. and Wiley, D.C., J. Mol. Biol., 212 (1990) 373.
Allen, F.H., Bellard, S.A., Brice, M.D., Cartwright, B.A. Doubleday, A., Higgs, H., Hummelink, T., Hummelink-Peters, B.G., Kennard, O., Motherwell, W.D.S., Rodgers, J.R. and Watson, D.G., Acta Crystallogr., B35 (1979) 2331.
Tronrud, D.E., Monzingo, A.F. and Matthews, B.W., Eur. J. Biochem., 157 (1986) 261.
Monzingo, A.F. and Matthews, B.W., Biochemistry, 23 (1984) 5724.
Holmes, M.A. and Matthews, B.W., Biochemistry, 20 (1981) 6912.
Holden, H.M., Tronrud, D.E., Monzingo, A.F., Weaver, L.H. and Matthews, B.W., Biochemistry, 26 (1987) 8542.
Tronrud, D.E., Holden, H.M. and Matthews, B.W., Science, 235 (1987) 571.
Debouck, C., AIDS Res. Hum. Retroviruses, 8 (1992) 153.
Wlodawer, A., Miller, M., Jaskolski, M., Sathyanarayana, B.K., Baldwin, E., Weber, I.T., Selk, I.M., Clawson, L., Schneider, J. and Kent, S.B.H., Science, 245 (1989) 616.
Miller, M., Schneider, J., Sathyanarayana, B.K., Toth, M.V., Marshall, G.R., Clawson, L., Selk, L., Kent, S.B.H. and Wlodawer, A., Science, 246 (1989) 1149.
Swain, A.L., Miller, M.M., Green, J., Rich, D.H., Kent, S.B.H. and Wlodawer, A., Proc. Natl. Acad. Sci. USA, 87 (1990) 8805.
Erickson, J., Neidhart, D.J., Van, Drie, J., Kempf, D.J., Wang, X.C., Norbeck, D.W., Plattner, J.J., Rittenhouse, J.W., Turon, M., Wideburg, N., Kohlbrenner, W.E., Simmer, R., Helfrich, R., Paul, D.A. and Knigge, M., Science, 249 (1990) 527.
Jaskolski, M., Tomasselli, A.G., Sawyer, T.K., Staples, D.G., Heinrikson, R.L., Schneider, J., Kent, S.B.H. and Wlodawer, A., Biochemistry, 30 (1991) 1600.
Bone, R., Vacca, J.P., Anderson, P.S. and Holloway, M.K., J. Am. Chem. Soc., 113 (1991) 9382.
Dreyer, G.B., Lambert, D.M., Meek, T.D., Carr, T.J., TomaszekJr., T.A., Fernandez, A.V., Bartus, H., Cacciavillani, E., Hassell, A.M., Mimmich, M., PettewayJr., S.R., Metcalf, B.W. and Lewis, M., Biochemistry, 31 (1992) 6646.
Dreyer, G.B., Boehm, J.C., Chenera, B., DesJarlais, R.L., Hassell, A.M., Meek, T.D., TomaszekJr., T.A. and Lewis, M., Biochemistry, 32 (1993) 937.
RusinkoIII, A., Skell, J.M., Balducci, R., McGarity, C.M. and Pearlman, R.S., CONCORD, University of Texas, Austin, TX and Tripos Associates, St. Louis, MO, 1988.
Sauter, N.K., Bednarski, M.D., Wurzburg, B.A., Hanson, J.E., Whitesides, G.M., Skehel, J.J. and Wiley, D.C., Biochemistry, 28 (1989) 8388.
Weiner, P.K. and Kollman, P.A., J. Comput. Chem., 2 (1981) 287.
Seibel, G.L., AMBER, Version 3.0A, University of California, San Francisco, CA, 1989.
Lawrence, M.C. and Davis, P.C., Protein Struct. Funct. Genet., 12 (1992) 31.
Shoichet, B.K., Bodian, D.L. and Kuntz, I.D., J. Comput. Chem., 13 (1992) 380.
Lam, P.Y.S., Jadhav, P.K., Eyermann, C.J., Hodge, C.N., Ru, Y., Bacheler, L.T., Meek, J.L., Otto, M.J., Rayner, M.M., Wong, Y.N., Chang, C.-H., Weber, P.C., Jackson, D.A., Sharpe, T.R., and Erickson-Viitanen, S., Science, 263 (1994) 380.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DesJarlais, R.L., Dixon, J.S. A shape- and chemistry-based docking method and its use in the design of HIV-1 protease inhibitors. J Computer-Aided Mol Des 8, 231–242 (1994). https://doi.org/10.1007/BF00126742
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00126742