Abstract
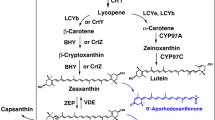
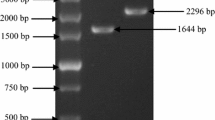
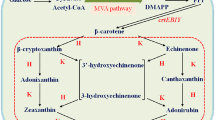
We succeeded in isolating a novel cDNA involved in astaxanthin biosynthesis from the green alga Haematococcus pluvialis, by an expression cloning method using an Escherichia coli transformant as a host that synthesizes β-carotene due to the Erwinia uredovora carotenoid biosynthesis genes. The cloned cDNA was shown to encode a novel enzyme, β-carotene ketolase (β-carotene oxygenase), which converted β-carotene to canthaxanthin via echinenone, through chromatographic and spectroscopic analysis of the pigments accumulated in an E. coli transformant. This indicates that the encoded enzyme is responsible for the direct conversion of methylene to keto groups, a mechanism that usually requires two different enzymatic reactions proceeding via a hydroxy intermediate. Northern blot analysis showed that the mRNA was synthesized only in the cyst cells of H. pluvialis. E. coli carrying the H. pluvialis cDNA and the E. uredovora genes required for zeaxanthin biosynthesis was also found to synthesize astaxanthin (3S, 3′S), which was identified after purification by a variety of spectroscopic methods.
Similar content being viewed by others
References
Andrewes AG, Borch G, Liaaen-Jensen S, Snatzke G: Animal carotenoids. 9. On the absolute configuration of astaxanthin and actinioerythrin. Acta Chem Scand B 28: 730–736 (1974).
Andrewes AG, Phaff HJ, Starr MP: Carotenoids of Phaffia rhodozyma, a red-pigmented fermenting yeast. Phytochemistry 15: 1003–1007 (1976).
Armstrong GA: Eubacteria show their true colors: genetics of carotenoid pigment biosynthesis from microbes to plants. J Bact 176: 4795–4802 (1994).
Boussiba S, Fan L, Vonshak A: Enhancement and determination of astaxanthin accumulation in green alga Haematococcus pluvialis. Meth Enzymol 213: 386–391 (1992).
Bouvier F, Hugueney P, d'Harlingue A, Kuntz M, Camara B: Xanthophyll biosynthesis in chromoplasts: isolation and molecular cloning of an enzyme catalyzing the conversion of 5,6-epoxycarotenoid into ketocarotenod. Plant J 6: 45–54 (1994).
Britton G: Biosynthesis of carotenoids. In: Goodwin TW (eds) Plant Pigments, pp. 133–182. Academic Press, London (1988).
Chomczynski P: A reagent for single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Bio Techniques 15: 532–536 (1993).
Cunningham FX Jr, Sun Z, Chamovitz D, Hirschberg J, Gantt E: Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp strain PCC7942. Plant Cell 6: 1107–1121 (1994).
Fraser PD, Misawa N, Linden H, Yamano S, Kobayashi K, Sandmann G: Expression in E. coli, purification and reactivation of the recombinant Erwinia uredovora phytoene desaturase. J Biol Chem 267: 19891–19895 (1992).
Grung M, D'Souza FML, Borowitzka M, Liaaen-Jensen S: Algal carotenoids 51. Secondary carotenoids 2. Haematococcus pluvialis aplanospores as a source of (3S, 3′S)-astaxanthin esters. J Appl Phycol 4: 165–171 (1992).
Johnson EA. An G-H: Astaxanthin from microbial sources. Crit Rev Biotechnol 11: 297–326 (1991).
Jyonouchi H, Zhang L, Tomita Y: Studies of immuno-modulating actions of carotenoids. II. Astaxanthin enhances in vivo antibody production to T-dependent antigens without facilitating polyclonal B-cell activation. Nutr Cancer 19: 269–280 (1993).
Kakizono T, Kobayashi M, Nagai S: Effect of carbon/nitrogen ratio on encystment accompanied with astaxanthin formation in green alga, Haematococcus pluvialis. J Ferment Bioeng 74: 403–405 (1992).
Kobayashi M, Kakizono T, Nagai S: Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol 59: 867–873 (1993).
Krinsky NI, Mathews-Roth MM, Taylor RF (eds): Carotenoids: Chemistry and Biology. Plenum Press, New York (1989).
Matsuno T: Xanthophylls as precursors of retinoids. Pure Appl Chem 63: 81–88 (1991).
Meyers SP: Developments in world aquaculture, feed formulations, and role of carotenoids. Pure Appl Chem 66: 1069–1076 (1994).
Miki W: Biological functions and activities of animal carotenoids. Pure Appl Chem 63: 141–146 (1991).
Miki W, Otaki N, Yokoyama A, Izumida H, Shimidzu N: Okadaxanthin, a novel C50-carotenoid from a bacterium, Pseudomonas sp. KK10206C associated with marine sponge, Halichondria okadai. Experientia 50: 684–686 (1994).
Misawa N, Kajiwara S, Kondo K, Yokoyama A, Satomi Y, Saito T, Miki W, Ohtani T: Canthaxanthin biosynthesis by the conversion of methylene to keto groups in a hydrocarbon β-carotene by a single gene. Biochem Biophys Res Com 209: 867–876 (1995).
Misawa N, Masamoto K, Hori T, Ohtani T, Böger P, Sandmann G: Expression of an Erwinia phytoene desaturase gene not only confers multiple resistance to herbicides interfering with carotenoid biosynthesis but also alters xanthophyll metabolism in transgenic plants. Plant J 6: 481–489 (1994).
Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K: Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol 172: 6704–6712 (1990).
Misawa N, Yamano S, Ikenaga H: Production of β-carotene in Zymomonas mobilis and Agrobacterium tumefaciens by introduction of the biosynthesis genes from Erwinia uredovora. Appl Environ Microbiol 57: 1847–1849 (1991).
Misawa N, Yamano S, Linden H, de Felipe MR, Lucas M, Ikenaga H, Sandmann G: Functional expression of the Erwinia uredovora carotenid biosynthesis gene crtI in transgenic plants showing an increase of β-carotene biosynthesis activity and resistance to the bleaching herbicide norflurazon. Plant J 4: 833–840 (1993).
Nelis NJ, Leenheer APD: Microbial sources of carotenoid pigments used in foods and feeds. J Appl Bact 70: 181–191 (1991).
Reddy CC, Hamilton GA, Madyastha KM: Biological Oxidation Systems, vol 1. Academic Press, San Diego (1990).
Rose RE: The nucleotide sequence of pACYC184. Nucl Acids Res 16: 355 (1988).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sandmann G: Carotenoid biosynthesis in microorganisms and plants. Eur J Biochem 223: 7–24 (1994).
Sandmann G, Misawa N: New functional assignment of the carotenogenic genes crtB and crtE with constructs of these genes from Erwinia species. FEMS Microbiol Lett 90: 253–258 (1992).
Tanaka T, Morishita Y, Suzui M, Kojima T, Okumura A, Mori H: Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin. Carcinogenesis 15: 15–19 (1994).
Yamano S, Ishii T, Nakagawa M, Ikenaga H, Misawa N: Metabolic engineering for production of β-carotene and lycopene in Saccharomyces cerevisiae. Biosci Biotech Biochem 58: 1112–1114 (1994).
Yokoyama A, Izumida H, Miki W: Production of astaxanthin and 4-ketozeaxanthin by the marine bacterium, Agrobacterium aurantiacum. Biosci Biotech Biochem 58: 1842–1844 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kajiwara, S., Kakizono, T., Saito, T. et al. Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis, and astaxanthin synthesis in Escherichia coli . Plant Mol Biol 29, 343–352 (1995). https://doi.org/10.1007/BF00043657
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00043657