Abstract
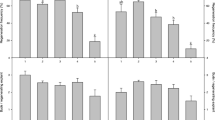
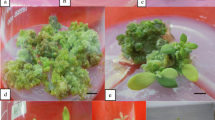
Explants composed of the epidermis and 4–9 layers of subepidermal cells were excised from internodes of Brassica napus L. ssp. oleifera cv. Westar and cultured on modified Murashige and Skoog (MS) medium. The three or four terminal internodes excised from plants at an early stage (before any flower buds had opened) were shown to be the best explant source. Both cytokinin and auxin were required for induction of shoot organogenesis. Of six auxins tested, only naphthaleneacetic acid (NAA) was effective in shoot bud initiation. All four cytokinins tested (when associated with 0.5 mgl-1 NAA) promoted organogenesis, but at differing frequencies. The highest shoot induction frequency was obtained at 10–15 mgl-1 benzyladenine (BA). The organogenic response was strongly affected by the nitrogen content of the medium. The best response was observed when NO3 - was the sole nitrogen source (supplied as KNO3) in the range 30–90 mM. Sucrose and glucose were equally supportive in shoot regeneration with the optimal levels at 0.12 M and 0.15 M, respectively. Shoots were rooted on medium free of growth regulators and mature plants were grown in the greenhouse. Plants were also recovered from leafy structures which differed morphologically and histologically from shoot buds.
Similar content being viewed by others
References
Bigot C (1976) Bourgeonnement in vitro a partir d'épiderme séparé de feuille de Bryophyllum daigremontianum (Crassulecées). Can J Bot 54: 852–867
Bilkey PC, Cocking EC (1981) Increased plant vigor by in vitro propagation of Saintpaulia ionantha Wendl. from subepidermal tissue. HortScience 16:643–644
Chlyah H (1974) Inter-tissue correlations in organ fragments. Plant Physiol 54: 341–348
Chlyah A, Tran Thanh Van M (1975) Differential reactivity in epidermal cells of Begonia rex excised and grown in vitro. Plant Physiol 35:16–20
Debergh PC (1983) Effects of agar brand and concentration on the tissue culture medium. Physiol Plant 59:210–276
Dietert MF, Barron SA, Yoder OC (1982) Effects of genotype on in vitro culture in the genus Brassica. Plant Sci Lett 26:233–240
Dunwell JM (1981) In vitro regeneration from excised leaf discs of three Brassica species. J. Exp Bot 32: 789–799
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gleddie S, Keller W, Setterfield G (1983) Somatic embryogenesis and plant regeneration from leaf explants and cell suspension of Solanum melongena (egg-plant). Can J Bot 61:656–666
Grant I, Harney PM (1982) In vitro propagation of rutabaga rootpieces. Can J Plant Sci 62:189–192
Hanh TT, Lie-Schricke H, Tran Thanh Van K (1981) Direct in vitro bud formation from fragments and thin cell layers of different organs of the winged bean (Psophocarpus tetragonolobus L. DC). Z Pflanzenphysiol 102:127–139
Hicks GS (1980) Patterns of organ development in plant tissue culture and the problem of organ determination. Bot Rev 46:1–23
Horák J, Lûstinec J, Mésiček J, Kaminek M, Poláčkov\.a D (1975) Regeneration of diploid and polyploid plants from the stem pith explants of diploid marrow stem kale (Brassica oleracea L). Ann Bot 39:371–377
Kartha KK, Gamborg OL, Constabel F (1974) In vitro plant formation from stem explants of rape (Brassica napus cv Zephyr). Physiol Plant 31:217–220
Kartha KK, Michayluk MR, Kao KN, Gamborg OL, Constabel F (1974) Callus formation and plant regeneration from mesophyll protoplasts of rape plants (Brassica napus L cv. Zephyr). Plant Sci Lett 3:265–271
Keller WA, Armstrong KC (1978) High frequency production of microspore derived plants from Brassica napus anther cultures. Z. Pflanzenphysiol 80:100–108
Kohlenbach HW, Wenzel G, Hoffmann F (1982) Regeneration of Brassica napus plantlets in cultures from isolated protoplasts of haploid stem embryos as compared with leaf protoplasts. Z Pflanzenphysiol 105:131–142
Larkin PJ, Scowcroft WR (1981) Somaclonal variation — a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Lazzeri PA, Dunwell JM (1984) In vitro shoot regeneration from seedling root segments of Brassica oleracea and Brassica napus cultivars. Ann of Bot 54:341–350
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. Z Pflanzenphysiol 105:427–434
Li L-c, Kohlenbach HW (1982) Somatic embryogenesis in quite a direct way in cultures of mesophyll protoplasts of Brassica napus (L.). Plant Cell Rep 1:209–211
Loh CS, Ingram DS (1982) Production of haploid plants from anther cultures and secondary embryoids of winter oilseed rape, Brassica napus ssp. oleifera. New Phytol 91:507–516
Margara J. Leydecker MT (1978) Differents types d'organogenese observés chez le colza, Brassica napus L var. oleifera Metzg. Applications à la multiplication végétative in vitro. C R Acad Sci Paris 287 17–20
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Pelletier G, Primard C, Vedel F, Chetrit P, Remy R, Rouselle P, Renard M (1983) Intergeneric cytoplasmic hybridization in Cruciferae by protoplast fusion. Mol Gen Genet 191:244–250
Rogozinska JH, Drozdowska L (1980) Organogenesis and plant formation from cotyledon and callus culture of rape. Acta Soc Bot Pol 48:5–20
Sacristan MD (1981) Regeneration of plants from long term callus cultures of haploid Brassica napus. Z Pflanzenzuchtg 86:248–253
Singh S, Chandra N (1984) Plant regeneration in callus and suspension cultures of Brassica campestris cv Yellow Sarson. Plant Cell Repts 3:1–4
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastructure Res 26:31–43
Stringam GR (1977) Regeneration in stem explants of haploid rapessed (Brassica napus L). Plant Sci Lett 9:115–119
Stringam GR (1979) Regeneration in leaf callus cultures of haploid rapeseed (Brassica napus L). Z Pflanzenphysiol 92:459–462
Thomas E, Hoffmann F, Potrykus I, Wenzel G (1976) Protoplast regeneration and stem embryogenesis of haploid androgenetic rape. Molec Gen Genet 145:245–247
Tran Thanh Van M (1973) Direct flower neoformation from superficial tissue of small explants of Nicotiana tabacum L. Planta 115:87–92
Tran Thanh Van M, Dien NT, Chlyah A (1974) Regulation of organogenesis in small explants of superficial tissue of Nicotiana tabacum L. Planta 119:149–159
Tran Thanh Van M (1980) Control of morphogenesis by inherent and exogenously applied factors in thin cell layers. Int Rev Cytol Suppl 11A:175–194
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klimaszewska, K., Keller, W.A. High frequency plant regeneration from thin cell layer explants of Brassica napus . Plant Cell Tiss Organ Cult 4, 183–197 (1985). https://doi.org/10.1007/BF00040193
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00040193