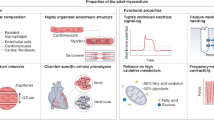
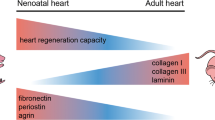
Abstract
Over the past decades, huge scientific efforts have been put into cardiac regeneration strategies. Although several strategies have been accepted for use in clinical practice, none has demonstrated great success in regenerating the cardiac tissue. Therefore, there are still significant challenges in repairing or regenerating cardiac tissue, which serves a predominantly biomechanical function. Furthermore, it is now evident that the mechanobiological interaction between cells and their mechanical environment is essential for tissue function and regeneration. Current cardiac regenerative strategies (i.e. cardiac cell therapy and tissue engineering) are based on administrating new healthy contractile cells within (or without) a healthy scaffold into the diseased cardiac environment. However, these strategies have widely omitted to restore the cellular mechanical environment present after cardiac injury. Therefore, the future cardiac regeneration strategies need to address the challenges of and questions on the role of biomechanics and mechanobiology in cardiac regeneration. This includes measurement and characterisation of multiscale mechanical properties of healthy and diseased cardiac tissue; development of in vitro and in silico models to understand the role of biomechanical factors in tissue regeneration; and translation of this information into the design of novel tissue-engineered strategies. In this chapter, we want to introduce the reader to the importance of mechanical considerations in the design of effective cardiac regeneration strategies.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
Living Heart Project | SIMULIA™ - Dassault Systèmes® (3ds.com)| SIMULIA™ - Dassault Systèmes® (3ds.com).
References
Mendis S, Puska P, Norrving B, World Health Organization, World Heart Federation, World Stroke Organization (2011) Global atlas on cardiovascular disease prevention and control. World Health Organization, Geneva, p vi, 155
Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV (2001) More “malignant” than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 3:315–322. https://doi.org/10.1016/S1388-9842(00)00141-0
Frangogiannis NG (2017) The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 127:1600–1612
Prabhu SD, Frangogiannis NG (2016) The biological basis for cardiac repair after myocardial infarction. Circ Res 119:91–112
Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB (2020) Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584:535–546. https://doi.org/10.1038/s41586-020-2612-2
Chin IL, Hool L, Choi YS (2019) A review of in vitro platforms for understanding cardiomyocyte mechanobiology. Front Bioeng Biotechnol 7:133. https://doi.org/10.3389/fbioe.2019.00133
Shapira-Schweitzer K, Seliktar D (2007) Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater 3:33–41. https://doi.org/10.1016/j.actbio.2006.09.003
Boothe SD, Myers JD, Pok S, Sun J, Xi Y, Nieto RM, Cheng J, Jacot JG (2016) The effect of substrate stiffness on cardiomyocyte action potentials. Cell Biochem Biophys 74:527–535. https://doi.org/10.1007/s12013-016-0758-1
D’Urso M, Kurniawan NA (2020) Mechanical and physical regulation of fibroblast–myofibroblast transition: from cellular mechanoresponse to tissue pathology. Front Bioeng Biotechnol 8:609653
Nakamura K, Murry CE (2019) Function follows form—a review of cardiac cell therapy. Circ J 83:2399–2412
Karantalis V, Difede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM (2014) Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (PROMETHEUS) trial. Circ Res 114:1302–1310. https://doi.org/10.1161/CIRCRESAHA.114.303180
Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher CA, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Puré E, Albelda SM, Epstein JA (2019) Targeting cardiac fibrosis with engineered T cells. Nature 573:430–433. https://doi.org/10.1038/s41586-019-1546-z
Fisher SA, Brunskill SJ, Doree C, Mathur A, Taggart DP, Martin-Rendon E (2014) Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev 4:CD007888
Tenreiro MF, Louro AF, Alves PM, Serra M (2021) Next generation of heart regenerative therapies: progress and promise of cardiac tissue engineering. NPJ Regen Med 61(6):1–17. https://doi.org/10.1038/s41536-021-00140-4
Cassino TR, Drowley L, Okada M, Beckman SA, Keller B, Tobita K, Leduc PRHJ (2012) Mechanical loading of stem cells for improvement of transplantation outcome in a model of acute myocardial infarction: the role of loading history. Tissue Eng Part A 18:1101–1108. https://doi.org/10.1089/TEN.TEA.2011.0285
Wu KH, Mo XM, Han ZC, Zhou B (2012) Cardiac cell therapy: pre-conditioning effects in cell-delivery strategies. Cytotherapy 14:260–266. https://doi.org/10.3109/14653249.2011.643780
Lee J, Henderson K, Massidda MW, Armenta-Ochoa M, Im BG, Veith A, Lee B-K, Kim M, Maceda P, Yoon E, Samarneh L, Wong M, Dunn AK, Kim J, Baker AB (2021) Mechanobiological conditioning of mesenchymal stem cells for enhanced vascular regeneration. Nat Biomed Eng 51(5):89–102. https://doi.org/10.1038/s41551-020-00674-w
Smits AM, van Laake LW, den Ouden K, Schreurs C, Szuhai K, van Echteld CJ, Mummery CL, Doevendans PA, Goumans M-J (2009) Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc Res 83:527–535. https://doi.org/10.1093/CVR/CVP146
Bax NA, van Marion MH, Shah B, Goumans MJ, Bouten CV, van der Schaft DW (2012) Matrix production and remodeling capacity of cardiomyocyte progenitor cells during in vitro differentiation. J Mol Cell Cardiol 53:497–508. https://doi.org/10.1016/J.YJMCC.2012.07.003
Montero P, Flandes-Iparraguirre M, Musquiz S, Pérez Araluce M, Plano D, Sanmartín C, Orive G, Gavira JJ, Prosper F, Mazo MM (2020) Cells, materials, and fabrication processes for cardiac tissue engineering. Front Bioeng Biotechnol 8:955
Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, Radisic M (2010) Challenges in cardiac tissue engineering. Tissue Eng Part B Rev 16:169. https://doi.org/10.1089/TEN.TEB.2009.0352
Radisic M (2015) Biomaterials for cardiac tissue engineering. Biomed Mater 10:030301. https://doi.org/10.1088/1748-6041/10/3/030301
Roshandel M, Dorkoosh F (2021) Cardiac tissue engineering, biomaterial scaffolds, and their fabrication techniques. Polym Adv Technol 32:2290–2305. https://doi.org/10.1002/PAT.5273
Majid QA, Fricker ATR, Gregory DA, Davidenko N, Hernandez Cruz O, Jabbour RJ, Owen TJ, Basnett P, Lukasiewicz B, Stevens M, Best S, Cameron R, Sinha S, Harding SE, Roy I (2020) Natural biomaterials for cardiac tissue engineering: a highly biocompatible solution. Front Cardiovasc Med 0:192. https://doi.org/10.3389/FCVM.2020.554597
Efraim Y, Sarig H, Cohen Anavy N, Sarig U, de Berardinis E, Chaw SY, Krishnamoorthi M, Kalifa J, Bogireddi H, Duc TV, Kofidis T, Baruch L, Boey FYC, Venkatraman SS, Machluf M (2017) Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater 50:220–233. https://doi.org/10.1016/j.actbio.2016.12.015
Li Z, Guan J (2011) Hydrogels for cardiac tissue engineering. Polymers (Basel) 3:740–761
Perea-Gil I, Gálvez-Montón C, Prat-Vidal C, Jorba I, Segú-Vergés C, Roura S, Soler-Botija C, Iborra-Egea O, Revuelta-López E, Fernández MA, Farré R, Navajas D, Bayes-Genis A (2018) Head-to-head comparison of two engineered cardiac grafts for myocardial repair: from scaffold characterization to pre-clinical testing. Sci Rep 8. https://doi.org/10.1038/s41598-018-25115-2
Prat-Vidal C, Rodríguez-Gómez L, Aylagas M, Nieto-Nicolau N, Gastelurrutia P, Agustí E, Gálvez-Montón C, Jorba I, Teis A, Monguió-Tortajada M, Roura S, Vives J, Torrents-Zapata S, Coca MI, Reales L, Cámara-Rosell ML, Cediel G, Coll R, Farré R, Navajas D, Vilarrodona A, García-López J, Muñoz-Guijosa C, Querol S, Bayes-Genis A (2020) First-in-human PeriCord cardiac bioimplant: scalability and GMP manufacturing of an allogeneic engineered tissue graft. EBioMedicine 54. https://doi.org/10.1016/J.EBIOM.2020.102729
Bejleri D, Davis ME (2019) Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv Healthc Mater 8:1801217. https://doi.org/10.1002/ADHM.201801217
Bouten CVC, Dankers PYW, Driessen-Mol A, Pedron S, Brizard AMA, Baaijens FPT (2011) Substrates for cardiovascular tissue engineering. Adv Drug Deliv Rev 63:221–241. https://doi.org/10.1016/J.ADDR.2011.01.007
Spaans S, Peter-Paul K, Fransen H, Schotman MJG, van der Wulp R, Lafleur RPM, Kluijtmans SGJM, Dankers PYW (2019) Supramolecular modification of a sequence-controlled collagen-mimicking polymer. Biomacromolecules 14:46. https://doi.org/10.1021/acs.biomac.9b00353
Mol EA, Lei Z, Roefs MT, Bakker MH, Goumans M, Doevendans PA, Dankers PYW, Vader P, Sluijter JPG (2019) Injectable supramolecular ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv Healthc Mater 8:1900847. https://doi.org/10.1002/adhm.201900847
Goor OJGM, Hendrikse SIS, Dankers PYW, Meijer EW (2017) From supramolecular polymers to multi-component biomaterials. Chem Soc Rev 46:6621–6637
Dwyer KD, Coulombe KLK (2021) Cardiac mechanostructure: Using mechanics and anisotropy as inspiration for developing epicardial therapies in treating myocardial infarction. Bioact Mater 6:2198–2220. https://doi.org/10.1016/J.BIOACTMAT.2020.12.015
Jarrell DK, Vanderslice EJ, VeDepo MC, Jacot JG (2020) Engineering myocardium for heart regeneration—advancements, considerations, and future directions. Front Cardiovasc Med 7:586261. https://doi.org/10.3389/fcvm.2020.586261
Jorba I, Mostert D, Hermans LHL, van der Pol A, Kurniawan NA, Bouten CVC (2021) In vitro methods to model cardiac mechanobiology in health and disease. Tissue Eng Part C Methods 27:139–151. https://doi.org/10.1089/TEN.TEC.2020.0342
Nemavhola F (2017) Biaxial quantification of passive porcine myocardium elastic properties by region. Eng Solid Mech 5:155–166. https://doi.org/10.5267/j.esm.2017.6.003
Andreu I, Luque T, Sancho A, Pelacho B, Iglesias-García O, Melo E, Farré R, Prósper F, Elizalde MR, Navajas D (2014) Heterogeneous micromechanical properties of the extracellular matrix in healthy and infarcted hearts. Acta Biomater 10:3235–3242. https://doi.org/10.1016/j.actbio.2014.03.034
Alcaraz J, Otero J, Jorba I, Navajas D (2018) Bidirectional mechanobiology between cells and their local extracellular matrix probed by atomic force microscopy. Semin Cell Dev Biol 73:71–81. https://doi.org/10.1016/j.semcdb.2017.07.020
Bers DM (2002) Cardiac excitation–contraction coupling. Nature 2002(415):198–205. https://doi.org/10.1038/415198a
Streeter D (1979) Gross morphology and fiber geometry of the heart
Arts T, Costa KD, Covell JW, McCulloch AD (2001) Relating myocardial laminar architecture to shear strain and muscle fiber orientation. Am J Physiol Heart Circ Physiol 280(5):H2222–H2229. https://doi.org/10.1152/AJPHEART.2001.280.5.H2222
Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH (1981) Left ventricular fibre architecture in man. Br Heart J 45:248–263. https://doi.org/10.1136/hrt.45.3.248
Burton RA, Plank G, Schneider JE, Grau V, Ahammer H, Keeling SL, Lee J, Smith NP, Gavaghan D, Trayanova N, Kohl P (2006) Three-dimensional models of individual cardiac histoanatomy: tools and challenges. Ann N Y Acad Sci 1080:301–319. https://doi.org/10.1196/ANNALS.1380.023
Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, Marwick TH, Thomas JD (2012) Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 98:1442–1448. https://doi.org/10.1136/HEARTJNL-2012-302353
Saito M, Okayama H, Yoshii T, Higashi H, Morioka H, Hiasa G, Sumimoto T, Inaba S, Nishimura K, Inoue K, Ogimoto A, Shigematsu Y, Hamada M, Higaki J (2012) Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur Hear J Cardiovasc Imaging 13:617–623. https://doi.org/10.1093/EJECHOCARD/JER318
Valente F, Gutierrez L, Rodríguez-Eyras L, Fernandez R, Montano M, Sao-Aviles A, Pineda V, Guala A, Cuéllar H, Evangelista A, Rodríguez-Palomares J (2020) Cardiac magnetic resonance longitudinal strain analysis in acute ST-segment elevation myocardial infarction: A comparison with speckle-tracking echocardiography. IJC Hear Vasc 29:100560. https://doi.org/10.1016/J.IJCHA.2020.100560
Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R (2009) Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Curr Cardiol Rev 5:133–148. https://doi.org/10.2174/157340309788166642
Pedrizzetti G, Claus P, Kilner PJ, Nagel E (2016) Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson 18:1–12. https://doi.org/10.1186/S12968-016-0269-7
Scatteia A, Baritussio A, Bucciarelli-Ducci C (2017) Strain imaging using cardiac magnetic resonance. Heart Fail Rev 224(22):465–476. https://doi.org/10.1007/S10741-017-9621-8
Mangion K, McComb C, Auger DA, Epstein FH, Berry C (2017) Magnetic resonance imaging of myocardial strain after acute ST-segment-elevation myocardial infarction: a systematic review. Circ Cardiovasc Imaging 10:e006498. https://doi.org/10.1161/CIRCIMAGING.117.006498
Huisman RM, Elzinga G, Westerhof N, Sipkema P (1980) Measurement of left ventricular wall stress. Cardiovasc Res 14:142–153. https://doi.org/10.1093/CVR/14.3.142
Guccione JM, McCulloch AD, Waldman LK (1991) Passive material properties of intact ventricular myocardium determined from a cylindrical model. J Biomech Eng 113:42–55. https://doi.org/10.1115/1.2894084
Feygin J, Hu Q, Swingen C, Zhang J (2008) Relationships between regional myocardial wall stress and bioenergetics in hearts with left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 294:H2313–H2321. https://doi.org/10.1152/AJPHEART.01288.2007
Walker JC, Ratcliffe MB, Zhang P, Wallace AW, Hsu EW, Saloner DA, Guccione JM (2008) Magnetic resonance imaging-based finite element stress analysis after linear repair of left ventricular aneurysm. J Thorac Cardiovasc Surg 135:1094–1102.e2. https://doi.org/10.1016/J.JTCVS.2007.11.038
Walker JC, Ratcliffe MB, Zhang P, Wallace AW, Fata B, Hsu EW, Saloner D, Guccione JM (2005) MRI-based finite-element analysis of left ventricular aneurysm. Am J Physiol Heart Circ Physiol 289:H692–H700. https://doi.org/10.1152/AJPHEART.01226.2004
Humphrey JD (2002) Cardiovascular solid mechanics, 1st edn. Springer, New York, pp 1–758. https://doi.org/10.1007/978-0-387-21576-1
Holmes JW, Borg TK, Covell JW (2005) Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng 7:223–253. https://doi.org/10.1146/ANNUREV.BIOENG.7.060804.100453
Fung Y-C (1993) Biomechanics, 2nd edn. Springer, New York, pp 1–568. https://doi.org/10.1007/978-1-4757-2257-4
Suki B (2014) Assessing the functional mechanical properties of bioengineered organs with emphasis on the lung. J Cell Physiol 229:1134–1140. https://doi.org/10.1002/jcp.24600
Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA (2005) Nonlinear elasticity in biological gels. Nature 435:191–194. https://doi.org/10.1038/nature03521
Demer LL, Yin FC (1983) Passive biaxial mechanical properties of isolated canine myocardium. J Physiol 339:615. https://doi.org/10.1113/JPHYSIOL.1983.SP014738
Novak VP, Yin FCP, Humphrey JD (1994) Regional mechanical properties of passive myocardium. J Biomech 27:403–412. https://doi.org/10.1016/0021-9290(94)90016-7
Dokos S, Smaill BH, Young AA, LeGrice IJ (2002) Shear properties of passive ventricular myocardium. Am J Physiol Heart Circ Physiol 283:2650–2659. https://doi.org/10.1152/AJPHEART.00111.2002
Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE (2008) Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci 121:3794–3802. https://doi.org/10.1242/jcs.029678
Fomovsky GM, Holmes JW (2010) Evolution of scar structure, mechanics, and ventricular function after myocardial infarction in the rat. Am J Physiol Heart Circ Physiol 298. https://doi.org/10.1152/ajpheart.00495.2009
Sirry MS, Butler JR, Patnaik SS, Brazile B, Bertucci R, Claude A, Mclaughlin R, Davies NH, Liao J, Franz T (2016) Characterisation of the mechanical properties of infarcted myocardium in the rat under biaxial tension and uniaxial compression. J Mech Behav Biomed Mater 63:252–264. https://doi.org/10.1016/j.jmbbm.2016.06.029
Brazile BL, Butler JR, Patnaik SS, Claude A, Prabhu R, Williams LN, Perez KL, Nguyen KT, Zhang G, Bajona P, Peltz M, Yang Y, Hong Y, Liao J (2021) Biomechanical properties of acellular scar ECM during the acute to chronic stages of myocardial infarction. J Mech Behav Biomed Mater 116:104342. https://doi.org/10.1016/J.JMBBM.2021.104342
Farré N, Jorba I, Torres M, Falcones B, Martí-Almor J, Farré R, Almendros I, Navajas D (2018) Passive stiffness of left ventricular myocardial tissue is reduced by ovariectomy in a post-menopause mouse model. Front Physiol 9. https://doi.org/10.3389/fphys.2018.01545
Jorba I, Uriarte JJ, Campillo N, Farré R, Navajas D (2017) Probing micromechanical properties of the extracellular matrix of soft tissues by atomic force microscopy. J Cell Physiol 232. https://doi.org/10.1002/jcp.25420
Zhang S, Sun A, Ma H, Yao K, Zhou N, Shen L, Zhang C, Zou Y, Ge J (2011) Infarcted myocardium-like stiffness contributes to endothelial progenitor lineage commitment of bone marrow mononuclear cells. J Cell Mol Med 15:2245–2261. https://doi.org/10.1111/J.1582-4934.2010.01217.X
Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL (2006) Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 290:2196–2203. https://doi.org/10.1152/AJPHEART.01017.2005
Notari M, Ventura-Rubio A, Bedford-Guaus SJ, Jorba I, Mulero L, Navajas D, Martí M, Raya Á (2018) The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci Adv 4:eaao5553. https://doi.org/10.1126/sciadv.aao5553
Garcia-Puig A, Mosquera JL, Jiménez-Delgado S, García-Pastor C, Jorba I, Navajas D, Canals F, Raya A (2019) Proteomics analysis of extracellular matrix remodeling during zebrafish heart regeneration. Mol Cell Proteomics 18(9):1745–1755. https://doi.org/10.1074/mcp.RA118.001193
Borin D, Pecorari I, Pena B, Sbaizero O (2018) Novel insights into cardiomyocytes provided by atomic force microscopy. Semin Cell Dev Biol 73:4–12. https://doi.org/10.1016/J.SEMCDB.2017.07.003
Liu J, Sun N, Bruce MA, Wu JC, Butte MJ (2012) Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes. PLoS One 7:e37559. https://doi.org/10.1371/JOURNAL.PONE.0037559
Lanzicher T, Martinelli V, Long CS, Del FG, Puzzi L, Borelli M, Mestroni L, Taylor MRG, Sbaizero O (2016) AFM single-cell force spectroscopy links altered nuclear and cytoskeletal mechanics to defective cell adhesion in cardiac myocytes with a nuclear lamin mutation. Nucleus 6:394–407. https://doi.org/10.1080/19491034.2015.1084453
Benech JC, Benech N, Zambrana AI, Rauschert I, Bervejillo V, Oddone N, Damián JP (2014) Diabetes increases stiffness of live cardiomyocytes measured by atomic force microscopy nanoindentation. Am J Physiol 307:C910–C919. https://doi.org/10.1152/AJPCELL.00192.2013
Lieber SC, Aubry N, Pain J, Diaz G, Kim S-J, Vatner SF (2004) Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am J Physiol Heart Circ Physiol 287:645–651. https://doi.org/10.1152/AJPHEART.00564.2003
Feinberg AW, Ripplinger CM, Van Der Meer P, Sheehy SP, Domian I, Chien KR, Parker KK (2013) Functional differences in engineered myocardium from embryonic stem cell-derived versus neonatal cardiomyocytes. Stem Cell Rep 1:387–396. https://doi.org/10.1016/j.stemcr.2013.10.004
Sheehy SP, Grosberg A, Qin P, Behm DJ, Ferrier JP, Eagleson MA, Nesmith AP, Krull D, Falls JG, Campbell PH, McCain ML, Willette RN, Hu E, Parker KK (2017) Toward improved myocardial maturity in an organ-on-chip platform with immature cardiac myocytes. Exp Biol Med (Maywood) 242:1643–1656. https://doi.org/10.1177/1535370217701006
Ariyasinghe NR, Reck CH, Viscio AA, Petersen AP, Lyra-Leite DM, Cho N, McCain ML (2017) Engineering micromyocardium to delineate cellular and extracellular regulation of myocardial tissue contractility. Integr Biol (United Kingdom) 9:730–741. https://doi.org/10.1039/c7ib00081b
Pasqualini FS, Agarwal A, O’Connor BB, Liu Q, Sheehy SP, Parker KK (2018) Traction force microscopy of engineered cardiac tissues. PLoS One 13:e0194706. https://doi.org/10.1371/journal.pone.0194706
McCain ML, Yuan H, Pasqualini FS, Campbell PH, Parker KK (2014) Matrix elasticity regulates the optimal cardiac myocyte shape for contractility. Am J Physiol Circ Physiol 306:H1525–H1539. https://doi.org/10.1152/ajpheart.00799.2013
Gopalan SM, Flaim C, Bhatia SN, Hoshijima M, Knoell R, Chien KR, Omens JH, McCulloch AD (2003) Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol Bioeng 81:578–587. https://doi.org/10.1002/bit.10506
Ribeiro AJS, Ang Y-S, Fu J-D, Rivas RN, Mohamed TMA, Higgs GC, Srivastava D, Pruitt BL (2015) Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci 112:12705–12710. https://doi.org/10.1073/pnas.1508073112
Ribeiro MC, Tertoolen LG, Guadix JA, Bellin M, Kosmidis G, D’Aniello C, Monshouwer-Kloots J, Goumans M-J, Wang Y, Feinberg AW, Mummery CL, Passier R (2015) Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro – correlation between contraction force and electrophysiology. Biomaterials 51:138–150. https://doi.org/10.1016/j.biomaterials.2015.01.067
Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schäfer H, Bishopric N, Wakatsuki T, Elson EL (1997) Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J 11:683–694. https://doi.org/10.1096/fasebj.11.8.9240969
Van Spreeuwel ACC, Bax NAM, Bastiaens AJ, Foolen J, Loerakker S, Borochin M, Van Der Schaft DWJ, Chen CS, Baaijens FPT, Bouten CVC (2014) The influence of matrix (an)isotropy on cardiomyocyte contraction in engineered cardiac microtissues. Integr Biol (United Kingdom) 6:422–429. https://doi.org/10.1039/c3ib40219c
van Spreeuwel ACC, Bax NAM, van Nierop BJ, Aartsma-Rus A, Goumans MJTH, Bouten CVC (2017) Mimicking cardiac fibrosis in a dish: fibroblast density rather than collagen density weakens cardiomyocyte function. J Cardiovasc Transl Res 10:116–127. https://doi.org/10.1007/s12265-017-9737-1
Jacot JG, McCulloch AD, Omens JH (2008) Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95:3479–3487. https://doi.org/10.1529/biophysj.107.124545
Mauretti A, Bax NAM, Van Marion MH, Goumans MJ, Sahlgren C, Bouten CVC (2016) Cardiomyocyte progenitor cell mechanoresponse unrevealed: Strain avoidance and mechanosome development. Integr Biol (United Kingdom) 8:991–1001. https://doi.org/10.1039/c6ib00117c
Wang EY, Rafatian N, Zhao Y, Lee A, Lai BFL, Lu RX, Jekic D, Davenport Huyer L, Knee-Walden EJ, Bhattacharya S, Backx PH, Radisic M (2019) Biowire model of interstitial and focal cardiac fibrosis. ACS Cent Sci 5:1146–1158. https://doi.org/10.1021/ACSCENTSCI.9B00052
Bracco Gartner TCL, Deddens JC, Mol EA, Magin Ferrer M, van Laake LW, Bouten CVC, Khademhosseini A, Doevendans PA, Suyker WJL, Sluijter JPG, Hjortnaes J (2019) Anti-fibrotic effects of cardiac progenitor cells in a 3D-model of human cardiac fibrosis. Front Cardiovasc Med 6:52. https://doi.org/10.3389/fcvm.2019.00052
Sadeghi AH, Shin SR, Deddens JC, Fratta G, Mandla S, Yazdi IK, Prakash G, Antona S, Demarchi D, Buijsrogge MP, Sluijter JPG, Hjortnaes J, Khademhosseini A (2017) Engineered 3D cardiac fibrotic tissue to study fibrotic remodeling. Adv Healthc Mater 6:1–14. https://doi.org/10.1002/adhm.201601434
Crocini C, Walker CJ, Anseth KS, Leinwand LA (2020) Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness. Prog Biophys Mol Biol 154:71–79. https://doi.org/10.1016/j.pbiomolbio.2019.04.008
Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML (2017) Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharmacol Sci 38:448–458
Lee S, Serpooshan V, Tong X, Venkatraman S, Lee M, Lee J, Chirikian O, Wu JC, Wu SM, Yang F (2017) Contractile force generation by 3D hiPSC-derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle-mimicking stiffness. Biomaterials 131:111–120. https://doi.org/10.1016/j.biomaterials.2017.03.039
Broughton KM, Russell B (2015) Cardiomyocyte subdomain contractility arising from microenvironmental stiffness and topography. Biomech Model Mechanobiol 14:589–602. https://doi.org/10.1007/s10237-014-0624-2
Corbin EA, Vite A, Peyster EG, Bhoopalam M, Brandimarto J, Wang X, Bennett AI, Clark AT, Cheng X, Turner KT, Musunuru K, Margulies KB (2019) Tunable and reversible substrate stiffness reveals a dynamic mechanosensitivity of cardiomyocytes. ACS Appl Mater Interfaces 11:20603–20614. https://doi.org/10.1021/acsami.9b02446
Spang MT, Christman KL (2018) Extracellular matrix hydrogel therapies: in vivo applications and development. Acta Biomater 68:1–14. https://doi.org/10.1016/J.ACTBIO.2017.12.019
Cramer MC, Badylak SF (2019) Extracellular matrix-based biomaterials and their influence upon cell behavior. Ann Biomed Eng 487(48):2132–2153. https://doi.org/10.1007/S10439-019-02408-9
Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee H-P, Lippens E, Duda GN, Mooney DJ (2016) Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 15:326–334. https://doi.org/10.1038/NMAT4489
Charrier EE, Pogoda K, Wells RG, Janmey PA (2018) Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat Commun 9:449. https://doi.org/10.1038/s41467-018-02906-9
Zhao H, Xu K, Zhu P, Wang C, Chi Q (2019) Smart hydrogels with high tunability of stiffness as a biomimetic cell carrier. Cell Biol Int 43:84–97. https://doi.org/10.1002/cbin.11091
Accardo JV, Kalow JA (2018) Reversibly tuning hydrogel stiffness through photocontrolled dynamic covalent crosslinks. Chem Sci 9:5987–5993. https://doi.org/10.1039/c8sc02093k
Wu X, Huang W, Wu WH, Xue B, Xiang D, Li Y, Qin M, Sun F, Wang W, Bin ZW, Cao Y (2018) Reversible hydrogels with tunable mechanical properties for optically controlling cell migration. Nano Res 11:5556–5565. https://doi.org/10.1007/s12274-017-1890-y
Bastings MMC, Koudstaal S, Kieltyka RE, Nakano Y, Pape ACH, Feyen DAM, van Slochteren FJ, Doevendans PA, Sluijter JPG, Meijer EW, Chamuleau SAJ, Dankers PYW (2014) A fast pH-switchable and self-healing supramolecular hydrogel carrier for guided, local catheter injection in the infarcted myocardium. Adv Healthc Mater 3:70–78. https://doi.org/10.1002/adhm.201300076
Stoppel WL, Kaplan DL, Black LD (2016) Electrical and mechanical stimulation of cardiac cells and tissue constructs. Adv Drug Deliv Rev 96:135–155
Yamane M, Matsuda T, Ito T, Fujio Y, Takahashi KAJ (2007) Rac1 activity is required for cardiac myocyte alignment in response to mechanical stress. Biochem Biophys Res Commun 353:1023–1027. https://doi.org/10.1016/J.BBRC.2006.12.144
Salameh A, Wustmann A, Karl S, Blanke K, Apel D, Rojas-Gomez D, Franke H, Mohr FW, Janousek J, Dhein S (2010) Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circ Res 106:1592–1602. https://doi.org/10.1161/CIRCRESAHA.109.214429
Debbi L, Drori S, Tzlil S (2018) The influence of the timing of cyclic load application on cardiac cell contraction. Front Physiol 0:917. https://doi.org/10.3389/FPHYS.2018.00917
Livne A, Bouchbinder E, Geiger B (2014) Cell reorientation under cyclic stretching. Nat Commun 51(5):1–8. https://doi.org/10.1038/ncomms4938
Tondon A, Hsu HJ, Kaunas R (2012) Dependence of cyclic stretch-induced stress fiber reorientation on stretch waveform. J Biomech 45:728–735. https://doi.org/10.1016/J.JBIOMECH.2011.11.012
Jungbauer S, Gao H, Spatz JP, Kemkemer R (2008) Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys J 95:3470–3478. https://doi.org/10.1529/BIOPHYSJ.107.128611
Boccafoschi F, Bosetti M, Gatti S, Cannas M (2007) Dynamic fibroblast cultures: response to mechanical stretching. Cell Adhes Migr 1:124–128. https://doi.org/10.4161/CAM.1.3.5144
Foolen J, Deshpande VS, Kanters FMW, Baaijens FPT (2012) The influence of matrix integrity on stress-fiber remodeling in 3D. Biomaterials 33:7508–7518. https://doi.org/10.1016/j.biomaterials.2012.06.103
Mostert D, Klouda L, van Turnhout MC, Kurniawan NA, Bouten CVC (2021) Cardiac fibroblast mechanoresponse guides anisotropic organization of hiPSC-derived cardiomyocytes in vitro. bioRxiv. https://doi.org/10.1101/2021.02.16.431369
Carlos-Oliveira M, Lozano-Juan F, Occhetta P, Visone R, Rasponi M (2021) Current strategies of mechanical stimulation for maturation of cardiac microtissues. Biophys Rev 13:717–727. https://doi.org/10.1007/S12551-021-00841-6/FIGURES/4
Mihic A, Li J, Miyagi Y, Gagliardi M, Li SH, Zu J, Weisel RD, Keller G, Li RK (2014) The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials 35:2798–2808. https://doi.org/10.1016/J.BIOMATERIALS.2013.12.052
Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, Cerino G, Redaelli A, Rasponi M (2016) Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 16:599–610. https://doi.org/10.1039/C5LC01356A
Llucià-Valldeperas A, Bragós R, Soler-Botija C, Roura S, Gálvez-Montón C, Prat-Vidal C, Perea-Gil I, Bayes-Genis A (2018) Unravelling the effects of mechanical physiological conditioning on cardiac adipose tissue-derived progenitor cells in vitro and in silico. Sci Rep 81(8):1–12. https://doi.org/10.1038/s41598-017-18799-5
LaBarge W, Mattappally S, Kannappan R, Fast VG, Pretorius D, Berry JL, Zhang J (2019) Maturation of three-dimensional, hiPSC-derived cardiomyocyte spheroids utilizing cyclic, uniaxial stretch and electrical stimulation. PLoS One 14:e0219442. https://doi.org/10.1371/JOURNAL.PONE.0219442
Kreutzer J, Viehrig M, Pölönen RP, Zhao F, Ojala M, Aalto-Setälä K, Kallio P (2020) Pneumatic unidirectional cell stretching device for mechanobiological studies of cardiomyocytes. Biomech Model Mechanobiol 19:291–303. https://doi.org/10.1007/S10237-019-01211-8/FIGURES/5
Dou W, Wang L, Malhi M, Liu H, Zhao Q, Plakhotnik J, Xu Z, Huang Z, Simmons CA, Maynes JT, Sun Y (2021) A microdevice platform for characterizing the effect of mechanical strain magnitudes on the maturation of iPSC-cardiomyocytes. Biosens Bioelectron 175:112875. https://doi.org/10.1016/J.BIOS.2020.112875
Lu K, Seidel T, Cao-Ehlker X, Dorn T, Batcha AMN, Schneider CM, Semmler M, Volk T, Moretti A, Dendorfer A, Tomasi R (2021) Progressive stretch enhances growth and maturation of 3D stem-cell-derived myocardium. Theranostics 11:6138–6153. https://doi.org/10.7150/THNO.54999
Kroll K, Chabria M, Wang K, Häusermann F, Schuler F, Polonchuk L (2017) Electro-mechanical conditioning of human iPSC-derived cardiomyocytes for translational research. Prog Biophys Mol Biol 130:212–222. https://doi.org/10.1016/J.PBIOMOLBIO.2017.07.003
Ovchinnikova E, Hoes M, Ustyantsev K, Bomer N, de Jong TV, van der Mei H, Berezikov E, van der Meer P (2018) Modeling human cardiac hypertrophy in stem cell-derived cardiomyocytes. Stem Cell Rep 10:794–807. https://doi.org/10.1016/J.STEMCR.2018.01.016
Viereck J, Bührke A, Foinquinos A, Chatterjee S, Kleeberger JA, Xiao K, Janssen-Peters H, Batkai S, Ramanujam D, Kraft T, Cebotari S, Gueler F, Beyer AM, Schmitz J, Bräsen JH, Schmitto JD, Gyöngyösi M, Löser A, Hirt MN, Eschenhagen T, Engelhardt S, Bär C, Thum T (2020) Targeting muscle-enriched long non-coding RNA H19 reverses pathological cardiac hypertrophy. Eur Heart J 41:3462–3474. https://doi.org/10.1093/EURHEARTJ/EHAA519
Nagaraju CK, Dries E, Gilbert G, Abdesselem M, Wang N, Amoni M, Driesen RB, Sipido KR (2019) Myofibroblast modulation of cardiac myocyte structure and function. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-45078-2
López-Canosa A, Perez-Amodio S, Yanac-Huertas E, Ordoño J, Rodriguez-Trujillo R, Samitier J, Castaño O, Engel E (2021) A microphysiological system combining electrospun fibers and electrical stimulation for the maturation of highly anisotropic cardiac tissue. Biofabrication 13:035047. https://doi.org/10.1088/1758-5090/ABFF12
Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Massé S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M (2013) Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods 10:781–787. https://doi.org/10.1038/nmeth.2524
Paez-Mayorga J, Hernández-Vargas G, Ruiz-Esparza GU, Iqbal HMN, Wang X, Zhang YS, Parra-Saldivar R, Khademhosseini A (2019) Bioreactors for cardiac tissue engineering. Adv Healthc Mater 8:1701504. https://doi.org/10.1002/ADHM.201701504
Abe H, Semba H, Takeda N (2017) The roles of hypoxia signaling in the pathogenesis of cardiovascular diseases. J Atheroscler Thromb 24:884–894. https://doi.org/10.5551/JAT.RV17009
D’Amario D, Migliaro S, Borovac JA, Restivo A, Vergallo R, Galli M, Leone AM, Montone RA, Niccoli G, Aspromonte N, Crea F (2019) Microvascular dysfunction in heart failure with preserved ejection fraction. Front Physiol 10:1347. https://doi.org/10.3389/FPHYS.2019.01347/BIBTEX
Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E, Zandstra PW (2009) Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng 102:493–507. https://doi.org/10.1002/BIT.22065
Correia C, Serra M, Espinha N, Sousa M, Brito C, Burkert K, Zheng Y, Hescheler J, Carrondo MJT, Šarić T, Alves PM (2014) Combining hypoxia and bioreactor hydrodynamics boosts induced pluripotent stem cell differentiation towards cardiomyocytes. Stem Cell Rev Rep 10:786–801. https://doi.org/10.1007/S12015-014-9533-0/FIGURES/7
Wang JH, Zhao L, Pan X, Chen NN, Chen J, Gong QL, Su F, Yan J, Zhang Y, Zhang SH (2016) Hypoxia-stimulated cardiac fibroblast production of IL-6 promotes myocardial fibrosis via the TGF-β1 signaling pathway. Lab Investig 968(96):839–852. https://doi.org/10.1038/labinvest.2016.65
Isenberg BC, Tranquillo RT (2003) Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng 31:937–949. https://doi.org/10.1114/1.1590662
Lu L, Mende M, Yang X, Körber HF, Schnittler HJ, Weinert S, Heubach J, Werner C, Ravens U, Lu L, Mende M, Yang X, Körber HF, Schnittler HJ, Weinert S, Heubach J, Werner C, Ravens U (2013) Design and validation of a bioreactor for simulating the cardiac niche: a system incorporating cyclic stretch, electrical stimulation, and constant perfusion. Tissue Eng Part A 19:403–414. https://doi.org/10.1089/TEN.TEA.2012.0135
Miklas JW, Nunes SS, Sofla A, Reis LA, Pahnke A, Xiao Y, Laschinger C, Radisic M (2014) Bioreactor for modulation of cardiac microtissue phenotype by combined static stretch and electrical stimulation. Biofabrication 6. https://doi.org/10.1088/1758-5082/6/2/024113
Wang B, Wang G, To F, Butler JR, Claude A, McLaughlin RM, Williams LN, de Jongh Curry AL, Liao J (2013) Myocardial scaffold-based cardiac tissue engineering: application of coordinated mechanical and electrical stimulations. Langmuir 29:11109–11117. https://doi.org/10.1021/LA401702W
van Kelle MAJ, Oomen PJA, Bulsink JA, Janssen-van den Broek MWJT, Lopata RGP, Rutten MCM, Loerakker S, Bouten CVC (2017) A bioreactor to identify the driving mechanical stimuli of tissue growth and remodeling. Tissue Eng Part C Methods 23:377–387. https://doi.org/10.1089/TEN.TEC.2017.0141
Savoji H, Mohammadi MH, Rafatian N, Toroghi MK, Wang EY, Zhao Y, Korolj A, Ahadian S, Radisic M (2019) Cardiovascular disease models: a game changing paradigm in drug discovery and screening. Biomaterials 198:3–26. https://doi.org/10.1016/j.biomaterials.2018.09.036
Mijailovich SM, Prodanovic M, Poggesi C, Geeves MA, Regnier M (2021) Multiscale modeling of twitch contractions in cardiac trabeculae. J Gen Physiol 153. https://doi.org/10.1085/JGP.202012604
Campbell SG, McCulloch AD (2011) Multi-scale computational models of familial hypertrophic cardiomyopathy: genotype to phenotype. J R Soc Interface 8:1550–1561. https://doi.org/10.1098/RSIF.2011.0184
Günter J, Wolint P, Bopp A, Steiger J, Cambria E, Hoerstrup SP, Emmert MY (2016) Microtissues in cardiovascular medicine: regenerative potential based on a 3d microenvironment. Stem Cells Int 2016. https://doi.org/10.1155/2016/9098523
Butler DL, Goldstein SA, Guldberg RE, Guo XE, Kamm R, Laurencin CT, McIntire LV, Mow VC, Nerem RM, Sah RL, Soslowsky LJ, Spilker RL, Tranquillo RT (2009) The impact of biomechanics in tissue engineering and regenerative medicine. Tissue Eng Part B Rev 15:477–484
Mihalef V, Passerini T, Mansi T (2020) Multi-scale models of the heart for patient-specific simulations. Artif Intell Comput Model Hear 2020:3–42. https://doi.org/10.1016/B978-0-12-817594-1.00011-5
Land S, Gurev V, Arens S, Augustin CM, Baron L, Blake R, Bradley C, Castro S, Crozier A, Favino M, Fastl TE, Fritz T, Gao H, Gizzi A, Griffith BE, Hurtado DE, Krause R, Luo X, Nash MP, Pezzuto S, Plank G, Rossi S, Ruprecht D, Seemann G, Smith NP, Sundnes J, Rice JJ, Trayanova N, Wang D, Wang ZJ, Niederer SA (2015) Verification of cardiac mechanics software: benchmark problems and solutions for testing active and passive material behaviour. Proc R Soc A Math Phys Eng Sci 471:20150641. https://doi.org/10.1098/RSPA.2015.0641
Urdeitx P, Doweidar MH (2020) A computational model for cardiomyocytes mechano-electric stimulation to enhance cardiac tissue regeneration. Mathematics. 8:1875. https://doi.org/10.3390/MATH8111875
Obbink-Huizer C, Foolen J, Oomens CWJ, Borochin M, Chen CS, Bouten CVC, FPT B (2014) Computational and experimental investigation of local stress fiber orientation in uniaxially and biaxially constrained microtissues. Biomech Model Mechanobiol 135(13):1053–1063. https://doi.org/10.1007/S10237-014-0554-Z
Obbink-Huizer C, Oomens CW, Loerakker S, Foolen J, Bouten CV, Baaijens FP (2014) Computational model predicts cell orientation in response to a range of mechanical stimuli. Biomech Model Mechanobiol 13:227–236. https://doi.org/10.1007/S10237-013-0501-4
Loerakker S, Obbink-Huizer C, Baaijens FPT (2013) A physically motivated constitutive model for cell-mediated compaction and collagen remodeling in soft tissues. Biomech Model Mechanobiol 135(13):985–1001. https://doi.org/10.1007/S10237-013-0549-1
Ristori T, Obbink-Huizer C, Oomens CW, Baaijens FP, Loerakker S (2016) Efficient computational simulation of actin stress fiber remodeling. Comput Methods Biomech Biomed Engin 19:1347–1358. https://doi.org/10.1080/10255842.2016.1140748
Ristori T, Notermans TMW, Foolen J, Kurniawan NA, Bouten CVC, Baaijens FPT, Loerakker S (2018) Modelling the combined effects of collagen and cyclic strain on cellular orientation in collagenous tissues. Sci Rep 81(8):1–14. https://doi.org/10.1038/s41598-018-26989-y
Davis J, Molkentin JD (2014) Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol 70:9–18. https://doi.org/10.1016/J.YJMCC.2013.10.019
Meshel AS, Wei Q, Adelstein RS, Sheetz MP (2005) Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol 72(7):157–164. https://doi.org/10.1038/ncb1216
Ristori T, Bouten CVC, Baaijens FPT, Loerakker S (2018) Predicting and understanding collagen remodeling in human native heart valves during early development. Acta Biomater 80:203–216. https://doi.org/10.1016/J.ACTBIO.2018.08.040
Emmert MY, Schmitt BA, Loerakker S, Sanders B, Spriestersbach H, Fioretta ES, Bruder L, Brakmann K, Motta SE, Lintas V, Dijkman PE, Frese L, Berger F, Baaijens FPT, Hoerstrup SP (2018) Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci Transl Med 10. https://doi.org/10.1126/SCITRANSLMED.AAN4587
Thavandiran N, Dubois N, Mikryukov A, Massé S, Beca B, Simmons CA, Deshpande VS, McGarry JP, Chen CS, Nanthakumar K, Keller GM, Radisic M, Zandstra PW (2013) Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc Natl Acad Sci U S A 110:E4698–E4707. https://doi.org/10.1073/PNAS.1311120110
Shim J, Grosberg A, Nawroth JC, Kit Parker K, Bertoldi K (2012) Modeling of cardiac muscle thin films: pre-stretch, passive and active behavior. J Biomech 45:832–841. https://doi.org/10.1016/J.JBIOMECH.2011.11.024
Toussaint N, Stoeck CT, Schaeffter T, Kozerke S, Sermesant M, Batchelor PG (2013) In vivo human cardiac fibre architecture estimation using shape-based diffusion tensor processing. Med Image Anal 17:1243–1255. https://doi.org/10.1016/J.MEDIA.2013.02.008
Cao Y, Miller MI, Winslow RL, Younes L (2005) Large deformation diffeomorphic metric mapping of vector fields. IEEE Trans Med Imaging 24:1216–1230. https://doi.org/10.1109/TMI.2005.853923
Genet M, Lee LC, Kuhl E, Guccione J (2014) Abaqus/Standard-based quantification of human cardiac mechanical properties. In: 2014 SIMULIA Community Conference (SCC2014), May 2014, Providence, Rhode Island, United States
Haddad SMH (2016) A novel composite material-based computational model for left ventricle biomechanics simulation. Electronic Thesis and Dissertation Repository
Acknowledgements
The authors would like to thank Gemma Burcet, MD from Hospital Universitari Vall d’Hebron and Institut de Diagnòstic per la Imatge (Barcelona, Spain), for the supply of magnetic resonance images and strain processing. This work is supported by the partners of ‘Regenerative Medicine Crossing Borders’ (RegMed XB) and by Health~Holland, Top Sector Life Sciences & Health. We also gratefully acknowledge funding from the Ministry of Education, Culture and Science for the Gravitation Program 024.003.103 ‘Materials-Driven Regeneration’ and Nederlandse Organisatie voor Wetenschappelijk Onderzoek for the NWO Open Competition Domain Science grant, OCENW.XS21.4.146.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Funding: This work was funded by 1) the partners of ‘Regenerative Medicine Crossing Borders’ and Health~Holland, Top Sector Life Sciences & Health (project Cardiac Moonshot), 2) the Ministry of Education, Culture and Science for the Gravitation Program ‘Materials-Driven Regeneration’ (grant number 024.003.103), and 3) by the Dutch Research Council (OCENW.XS21.4.146).
Conflict of Interest: All authors declare they have no conflict of interest.
Ethical Approval: This chapter does not contain any studies with animals performed by any of the authors. For Fig. 2d, g informed consent was obtained from the patients.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jorba, I., Nikolic, M., Bouten, C.V.C. (2023). Mechanical Considerations of Myocardial Tissue and Cardiac Regeneration. In: Hecker, M., Duncker, D.J. (eds) Cardiac Mechanobiology in Physiology and Disease. Cardiac and Vascular Biology, vol 9. Springer, Cham. https://doi.org/10.1007/978-3-031-23965-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-23965-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23964-9
Online ISBN: 978-3-031-23965-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)