Ionic Liquid-Incorporated Zn-Ion Conducting Polymer Electrolyte Membranes
Abstract
:1. Introduction
2. Materials and Methods
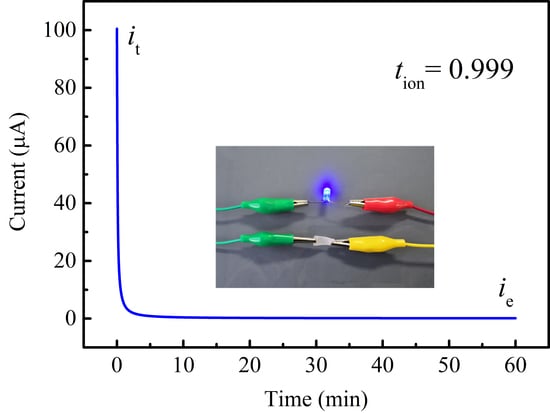
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Helbig, C.; Bradshaw, A.M.; Wietschel, L.; Thorenz, A.; Tuma, A. Supply risks associated with lithium-ion battery materials. J. Clean. Prod. 2018, 172, 274–286. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Verma, V.; Kumar, S.; Manalastas, W.; Satish, R.; Srinivasan, M. Progress in Rechargeable Aqueous Zinc- and Aluminum-Ion Battery Electrodes: Challenges and Outlook. Adv. Sustain. Syst. 2019, 3, 1800111. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Tan, H.; Chao, D.L.; Fan, H.J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Lee, B.; Yoon, C.S.; Lee, H.R.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Electrochemically-induced reversible transition from the tunneled to layered polymorphs of manganese dioxide. Sci. Rep. 2015, 4, 6066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-Lubricated Intercalation in V2O5 center dot nH(2)O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef] [PubMed]
- Trocoli, R.; Mantia, F.L. An aqueous zinc-ion battery based on copper hexacyanoferrate. ChemSusChem 2015, 8, 481–485. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 Nanosheet-Based Aqueous Zn Ion Battery Cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Kaveevivitchai, W.; Manthiram, A. High-capacity Zinc-ion Storage in An Open-Tunnel Oxide for Aqueous and Nonaqueous Zn-ion Batteries. J. Mater. Chem. A 2016, 4, 18737–18741. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.W.; Liu, J.; Gong, Y.D.; Wilkinson, D.P.; Zhang, J.J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.L.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H.S. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.X.; Wang, Y.; Song, M.M.; Long, L.; Siyal, S.H.; Yang, X.P.; Sui, G. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2019, 37, 126–142. [Google Scholar] [CrossRef] [Green Version]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO-alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Huang, Y.X.; Huang, Y.; Liu, B.; Cao, H.J.; Zhao, L.; Song, A.; Lin, Y.H.; Wang, M.S.; Li, X.; Zhang, Z.P. Gel polymer electrolyte based on p(acrylonitrile-maleic anhydride) for lithium ion battery. Electrochim. Acta 2018, 286, 242–251. [Google Scholar] [CrossRef]
- Singh, S.K.; Shalu, B.L.; Gupta, H.; Singh, V.K.; Tripathi, A.K.; Verma, Y.L.; Singh, R.K. Improved electrochemical performance of EMIMFSI ionic liquid based gel polymer electrolyte with temperature for rechargeable lithium battery. Energy 2018, 150, 890–900. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Conductivity study of porous plasticized polymer electrolytes based on poly(vinylidene fluoride)-A comparison with polypropylene separators. J. Electrochem. Soc. 2000, 147, 3219–3225. [Google Scholar] [CrossRef]
- Li, M.S.; Liao, Y.H.; Liu, Q.Y.; Xu, J.X.; Sun, P.; Shi, H.N.; Li, W.S. Application of the imidazolium ionic liquid based nano-particle decorated gel polymer electrolyte for high safety lithium ion battery. Electrochim. Acta 2018, 284, 188–201. [Google Scholar] [CrossRef]
- Francis, C.F.J.; Kyratzis, I.L.; Best, A.S. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- He, C.F.; Liu, J.Q.; Cui, J.Q.; Li, J.; Wu, X.F. A gel polymer electrolyte based on Polyacrylonitrile/organic montmorillonite membrane exhibiting dense structure for lithium ion battery. Solid State Ion. 2018, 315, 102–110. [Google Scholar] [CrossRef]
- Guo, Q.P.; Han, Y.; Wang, H.; Xiong, S.Z.; Liu, S.K.; Zheng, C.M.; Xie, K. Preparation and characterization of nanocomposite ionic liquid-based gel polymer electrolyte for safe applications in solid-state lithium battery. Solid State Ion. 2018, 321, 48–54. [Google Scholar] [CrossRef]
- Saroj, A.L.; Singh, R.K. Thermal, dielectric and conductivity studies on PVA/Ionic liquid [EMIM][EtSO4] based polymer electrolytes. J. Phys. Chem. Solids 2012, 73, 162–168. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Arof, A.K. Investigation of electrical and electrochemical properties of PVDF-based polymer electrolytes. J. Power Sources 2004, 132, 229–234. [Google Scholar] [CrossRef]
- Mccrum, N.G. A Study of Internal Friction in Copolymers of Tetrafluoroethylene and Hexafluoropropylene. Makromolekul. Chem. 1959, 34, 50–66. [Google Scholar] [CrossRef]
- Abbent, S.; Plestil, J.; Hlavata, D.; Lindgren, J.; Tegenfeldt, J.; Wendsjo, A. Crystallinity and morphology of PVdF-HFP-based gel electrolytes. Polymer 2001, 42, 1407–1416. [Google Scholar] [CrossRef]
- Long, L.Z.; Wang, S.J.; Xiao, M.; Meng, Y.Z. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Das, S.; Kumar, P.; Dutta, K.; Kundu, P.P. Partial Sulfonation of PVdF-co-HFP: A Preliminary Study and Characterization for Application in Direct Methanol Fuel Cell. Appl. Energy 2014, 113, 169–177. [Google Scholar] [CrossRef]
- Hariprasad, R.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. SPVdF-HFP/SGO nanohybrid proton exchange membrane for the applications of direct methanol fuel cells. J. Dispers. Sci. Technol. 2019, 1–13. [Google Scholar] [CrossRef]
- Gnana, K.G.; Kim, A.R.; Nahm, K.S.; Yoo, D.J. High proton conductivity and low fuel crossover of polyvinylidene fluoride–hexafluoro propylene–silica sulfuric acid composite membranes for direct methanol fuel cells. Curr. Appl. Phys. 2011, 11, 896–902. [Google Scholar] [CrossRef]
- Kim, A.R.; Gabunada, J.C.; Yoo, D.J. Sulfonated fluorinated block copolymer containing naphthalene unit/sulfonated polyvinylidene-co-hexafluoropropylene/functionalized silicon dioxide ternary composite membrane for low-humidity fuel cell applications. Colloid Polym. Sci. 2018, 296, 1891–1903. [Google Scholar] [CrossRef]
- Kumar, D.; Kanchan, D.K. Dielectric and electrochemical studies on carbonate free Na-ion conducting electrolytes for sodium-sulfur batteries. J. Energy Storage 2019, 22, 44–49. [Google Scholar] [CrossRef]
- Tang, X.; Muchakayala, R.; Song, S.H.; Zhang, Z.Y.; Polu, A.R. A study of structural; electrical and electrochemical properties of PVdF-HFP gel polymer electrolyte films for magnesium ion battery applications. J. Ind. Eng. Chem. 2016, 37, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.H.; Khanam, Z.; Muchakayala, R.; Song, S.H. Fabrication and characterization of Zn-ion-conducting solid polymer electrolyte films based on PVdF-HFP/Zn(Tf)2 complex system. J. Mater. Sci. Mater. Electron. 2020, 31, 6160–6173. [Google Scholar] [CrossRef]
- Rathika, R.; Austin, S.S. Effect of ionic liquid 1-ethyl-3-methylimidazolium hydrogen sulfate on zinc-ion dynamics in PEO/PVdF blend gel polymer electrolytes. Ionics 2018, 25, 1137–1146. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhao, Z.J.; Song, S.H.; Ma, Q.; Liu, R.C. High Performance Poly(vinyl alcohol)-Based Li-Ion Conducting Gel Polymer Electrolyte Films for Electric Double-Layer Capacitors. Polymers 2018, 10, 1179. [Google Scholar] [CrossRef] [Green Version]
- Liew, C.W.; Arifin, K.H.; Kawamura, J.; Iwai, Y.; Ramesh, S.; Arof, A.K. Effect of halide anions in ionic liquid added poly(vinyl alcohol)-based ion conductors for electrical double layer capacitors. J. Non-Cryst. Solids 2017, 458, 97–106. [Google Scholar] [CrossRef]
- Senthil, K.P.; Sakunthala, A.; Reddy, M.V.; Prabu, M. Structural, morphological, electrical and electrochemical study on plasticized PVdF-HFP/PEMA blended polymer electrolyte for lithium polymer battery application. Solid State Ion. 2018, 319, 256–265. [Google Scholar] [CrossRef]
- Tsao, C.H.; Kuo, P.L. Poly(dimethylsiloxane) hybrid gel polymer electrolytes of a porous structure for lithium ion battery. J. Membr. Sci. 2015, 489, 36–42. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.Y.; Lei, G.; Liu, H.T. Ionic liquid-gelled polyvinylidene fluoride/polyvinyl acetate polymer electrolyte for solid supercapacitor. Chem. Eng. J. 2014, 258, 320–326. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Mohamed, N.S.; Isa, M.I.N. Investigation of the Ionic Conduction Mechanism in Carboxymethyl Cellulose/Chitosan Biopolymer Blend Electrolyte Impregnated with Ammonium Nitrate. Int. J. Polym. Anal. Charact. 2015, 20, 491–503. [Google Scholar] [CrossRef]
- Tang, J.W.; Muchakayala, R.; Song, S.H.; Wang, M.; Kumar, K.N. Effect of EMIMBF4 ionic liquid addition on the structure and ionic conductivity of LiBF4-complexed PVdF-HFP polymer electrolyte films. Polym. Test. 2016, 50, 247–254. [Google Scholar] [CrossRef]
- Monisha, S.; Selvasekarapandian, S.; Mathavan, T.; Milton, F.B.A.; Manoharan, S.; Karthikeyan, S. Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J. Mater. Sci. Mater. Electron. 2016, 27, 9314–9324. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Saadiah, M.A. Ionic conduction study of enhanced amorphous solid bio-polymer electrolytes based carboxymethyl cellulose doped NH4Br. J. Non-Cryst. Solids 2018, 497, 19–29. [Google Scholar] [CrossRef]
- Unnisa, C.N.; Chitra, S.; Selvasekarapandian, S.; Monisha, S.; Devi, G.N.; Moniha, V.; Hema, M. Development of poly(glycerol suberate) polyester (PGS)–PVA blend polymer electrolytes with NH4SCN and its application. Ionics 2018, 24, 1979–1993. [Google Scholar] [CrossRef]
- Ravi, M.; Song, S.H.; Wang, J.W.; Wang, T.; Nadimicherla, R. Ionic liquid incorporated biodegradable gel polymer electrolyte for lithium ion battery applications. J. Mater. Sci. Mater. Electron. 2016, 27, 1370–1377. [Google Scholar] [CrossRef]
- Wang, J.W.; Song, S.H.; Muchakayala, R.; Hu, X.C.; Liu, R.C. Structural, electrical, and electrochemical properties of PVA-based biodegradable gel polymer electrolyte membranes for Mg-ion battery applications. Ionics 2017, 23, 1759–1769. [Google Scholar] [CrossRef]
- Hu, X.C.; Muchakayala, R.; Song, S.H.; Wang, J.W.; Chen, J.J.; Tan, M.L. Synthesis and optimization of new polymeric ionic liquid poly(diallydimethylammonium) bis(trifluoromethane sulfonyl)imde based gel electrolyte films. Int. J. Hydrogen Energy 2018, 43, 3741–3749. [Google Scholar] [CrossRef]
- Singh, S.K.; Gupta, H.; Balo, L.; Singh, V.K.; Tripathi, A.K.; Verma, Y.L.; Singh, R.K. Electrochemical characterization of ionic liquid based gel polymer electrolyte for lithium battery application. Ionics 2018, 24, 1895–1906. [Google Scholar] [CrossRef]
- Vraneš, M.; Cvjetićanin, N.; Papović, S.; Šarac, B.; Prislan, I.; Megušar, P.; Gadžurić, S.; Rogač, B.M. Electrical, electrochemical and thermal properties of the ionic liquid + lactone binary mixtures as the potential electrolytes for lithium-ion batteries. J. Mol. Liq. 2017, 243, 52–60. [Google Scholar] [CrossRef]
- Wang, J.W.; Chen, G.P.; Song, S.H. Na-ion conducting gel polymer membrane for flexible supercapacitor application. Electrochim. Acta 2020, 330, 135322. [Google Scholar] [CrossRef]
- Pal, P.; Ghosh, A. Highly efficient gel polymer electrolytes for all solid-state electrochemical charge storage devices. Electrochim. Acta 2018, 278, 137–148. [Google Scholar] [CrossRef]
- Jie, J.; Liu, Y.L.; Cong, L.N.; Zhang, B.H.; Lu, W.; Zhang, X.M.; Liu, J.; Xie, H.M.; Sun, L.Q. High-performance PVDF-HFP based gel polymer electrolyte with a safe solvent in Li metal polymer battery. J. Energy Chem. 2020, 49, 80–88. [Google Scholar] [CrossRef]
- Tao, C.; Gao, M.H.; Yin, B.H.; Li, B.; Huang, Y.P.; Xu, G.; Bao, J.J. A promising TPU/PEO blend polymer electrolyte for all-solid-state lithium ion batteries. Electrochim. Acta 2017, 257, 31–39. [Google Scholar] [CrossRef]







| Samples | Ionic Conductivities (S cm−1) |
|---|---|
| SPE-Zn | 2.44 × 10−5 |
| ILPE-Zn-1 | 4.03 × 10−5 |
| ILPE-Zn-2 | 6.56 × 10−5 |
| ILPE-Zn-3 | 7.15 × 10−5 |
| ILPE-Zn-4 | 1.44 × 10−4 |
| ILPE-Zn-5 | 1.18 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Ahmed, S.; Khanam, Z.; Wang, T.; Song, S. Ionic Liquid-Incorporated Zn-Ion Conducting Polymer Electrolyte Membranes. Polymers 2020, 12, 1755. https://doi.org/10.3390/polym12081755
Liu J, Ahmed S, Khanam Z, Wang T, Song S. Ionic Liquid-Incorporated Zn-Ion Conducting Polymer Electrolyte Membranes. Polymers. 2020; 12(8):1755. https://doi.org/10.3390/polym12081755
Chicago/Turabian StyleLiu, Jianghe, Sultan Ahmed, Zeba Khanam, Ting Wang, and Shenhua Song. 2020. "Ionic Liquid-Incorporated Zn-Ion Conducting Polymer Electrolyte Membranes" Polymers 12, no. 8: 1755. https://doi.org/10.3390/polym12081755