Nanostructured Lipid Carriers to Mediate Brain Delivery of Temazepam: Design and In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Fabrication of Temazepam-Loaded Nanostructured Lipid Carriers
2.4. Experimental Design
Optimization of Data
2.5. Characterization of Temazepam-Loaded Nanostructured Lipid Carriers
2.5.1. Particle Size and Zeta Potential
2.5.2. Encapsulation Efficiency
2.5.3. Morphology
2.5.4. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5.5. Differential Scanning Calorimetry (DSC)
2.5.6. X-ray Diffraction (XRD)
2.5.7. In Vitro Release Study
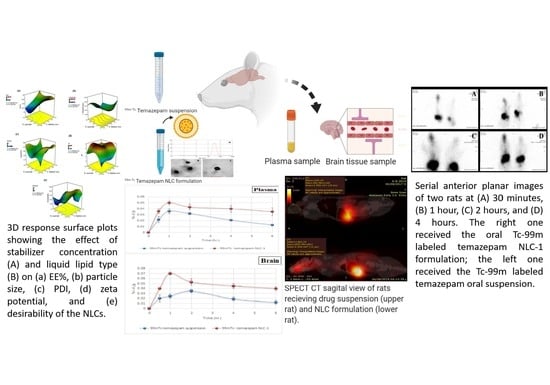
2.6. In Vivo Study
2.6.1. Radiolabeling of Temazepam and NLC Formulation with Technitium-99m
2.6.2. Gamma Scintigraphy Imaging
2.6.3. Biodistribution Study
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fabrication and Optimization of Temazepam Loaded NLCs
3.1.1. Effect of Stabilizer Concentration on Particle Size
3.1.2. Effect of Stabilizer Concentration on Encapsulation Efficiency Percent (EE%)
3.1.3. Effect of Stabilizer Concentration on Zeta Potential
3.1.4. Effect of Type of Liquid Lipid
3.1.5. Effect of Type of Liquid Lipid on Particle Size
3.1.6. Effect of Type of Liquid Lipid on Zeta Potential
3.1.7. Effect of Type of Liquid Lipid on Encapsulation Efficiency (EE%)
3.1.8. Effect of Type of Liquid Lipid on PDI
3.1.9. Selection of Optimized NLC Formulation
3.2. Characterization of Temazepam-Loaded NLC Formulation
3.2.1. FTIR Analysis
3.2.2. DSC Analysis
3.2.3. X-ray Diffraction
3.2.4. Morphological Analysis
3.2.5. In Vitro Release Profile of Temazepam Loaded NLC Formulation
3.3. In Vivo Studies
3.3.1. Gamma Scintigraphic Imaging
3.3.2. Pharmacokinetics and Brain Distribution
4. Conclusions
5. Recommendations and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481. [Google Scholar] [CrossRef]
- Guerra, M.; Blázquez, J.; Rodríguez, E. Blood–brain barrier and foetal-onset hydrocephalus, with a view on potential novel treatments beyond managing CSF flow. Fluids Barriers CNS 2017, 14, 19. [Google Scholar] [CrossRef]
- Masserini, M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, U.; Knoflach, F. Beyond classical benzodiazepines: Novel therapeutic potential of GABA A receptor subtypes. Nat. Rev. Drug Discov. 2011, 10, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, U.; Crestani, F.; Benke, D.; Brünig, I.; Benson, J.A.; Fritschy, J.-M.; Martin, J.R.; Bluethmann, H.; Möhler, H. erratum: Benzodiazepine actions mediated by specific γ-aminobutyric acid A receptor subtypes. Nature 2000, 404, 629. [Google Scholar] [CrossRef]
- Hosie, H.; Nimmo, W. Temazepam absorption in patients before surgery. Br. J. Anaesth. 1991, 66, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.J.; Pu, Q.L.; Yang, S.K. Hydrolysis of temazepam in simulated gastric fluid and its pharmacological consequence. J. Pharm. Sci. 1994, 83, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9, S3. [Google Scholar] [CrossRef] [Green Version]
- Griffin, C.E.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system–mediated effects. Ochsner J. 2013, 13, 214–223. [Google Scholar]
- Halliday, N.; Dundee, J.; Carlisle, R.; McClean, E. Iv temazepam: Theoretical and clinical considerations. BJA Br. J. Anaesth. 1987, 59, 465–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muggetti, L.; Zurlo, M.; Martini, A.; Civaroli, P.; De Ponti, R. Preformulation activities of intranasal dosage forms of temazepam. Int. J. Pharm. 1996, 136, 81–88. [Google Scholar] [CrossRef]
- Hanff, L.; Rutten, W. Pharmacokinetic aspects of rectal formulations of temazepam. Pharm. World Sci. 1996, 18, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickup, M.; Rogers, M.; Launchbury, A. Temazepam elixir: Comparative bioavailability with a capsule formulation. Int. J. Pharm. 1984, 22, 311–319. [Google Scholar] [CrossRef]
- Reddy, R. Formulation and In Vitro Evaluation of Temazepam Oral Dispersible Tablets. Asian J. Pharm. Anal. Med. Chem. 2014, 2, 1–8. [Google Scholar]
- Verheyen, S.; Blaton, N.; Kinget, R.; Van den Mooter, G. Mechanism of increased dissolution of diazepam and temazepam from polyethylene glycol 6000 solid dispersions. Int. J. Pharm. 2002, 249, 45–58. [Google Scholar] [CrossRef]
- Bellavance, M.-A.; Blanchette, M.; Fortin, D. Recent advances in blood–brain barrier disruption as a CNS delivery strategy. AAPS J. 2008, 10, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Minn, A.; Leclerc, S.; Heydel, J.-M.; Minn, A.-L.; Denizot, C.; Cattarelli, M.; Netter, P.; Gradinaru, D. Drug transport into the mammalian brain: The nasal pathway and its specific metabolic barrier. J. Drug Target. 2002, 10, 285–296. [Google Scholar] [CrossRef]
- Omar, M.M.; Eleraky, N.E.; El Sisi, A.M.; Ali Hasan, O. Development and Evaluation of in-situ Nasal Gel Formulations of Nanosized Transferosomal Sumatriptan: Design, Optimization, in vitro and in vivo Evaluation. Drug Des. Dev. Ther. 2019, 13, 4413–4430. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D. The next big things are tiny. Lancet Neurol. 2013, 12, 31–32. [Google Scholar] [CrossRef]
- Re, F.; Gregori, M.; Masserini, M. Nanotechnology for neurodegenerative disorders. Maturitas 2012, 73, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinís, M.Á.; Gascón, A.R.; del Pozo-Rodríguez, A.; des Rieux, A.; Préat, V. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J. Control. Release 2013, 166, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, C.-Y.; Li, N.; Wang, M.; Zhang, X.-N.; Pan, W.-S.; Peng, J.-J.; Pan, Y.-S.; Tang, X. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int. J. Pharm. 2010, 394, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Martin-Banderas, L.; Holgado, M.A.; Venero, J.L.; Alvarez-Fuentes, J.; Fernández-Arévalo, M. Nanostructures for drug delivery to the brain. Curr. Med. Chem. 2011, 18, 5303–5321. [Google Scholar] [CrossRef]
- Khan, S.; Baboota, S.; Ali, J.; Khan, S.; Narang, R.S.; Narang, J.K. Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs. Int. J. Pharm. Investig. 2015, 5, 182. [Google Scholar]
- Chen, Y.; Pan, L.; Jiang, M.; Li, D.; Jin, L. Nanostructured lipid carriers enhance the bioavailability and brain cancer inhibitory efficacy of curcumin both in vitro and in vivo. Drug Deliv. 2016, 23, 1383–1392. [Google Scholar]
- Lim, W.M.; Rajinikanth, P.S.; Mallikarjun, C.; Kang, Y.B. Formulation and delivery of itraconazole to the brain using a nanolipid carrier system. Int. J. Nanomed. 2014, 9, 2117. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-J.; Wu, P.-C.; Huang, Y.-B.; Chang, J.-S.; Lin, C.-L.; Tsai, Y.-H.; Fang, J.-Y. Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. Int. J. Pharm. 2012, 423, 461–470. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, L.; Zhou, L.; Zhang, Z.H.; Cao, W.; Wu, Q. Effect of cell-penetrating peptide-coated nanostructured lipid carriers on the oral absorption of tripterine. Int. J. Nanomed. 2012, 7, 4581. [Google Scholar]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2011; pp. 63–70. [Google Scholar]
- El-Gazayerly, O.N.; Hikal, A.H. Preparation and evaluation of acetazolamide liposomes as an ocular delivery system. Int. J. Pharm. 1997, 158, 121–127. [Google Scholar] [CrossRef]
- Devaraj, G.N.; Parakh, S.; Devraj, R.; Apte, S.; Rao, B.R.; Rambhau, D. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J. Colloid Interface Sci. 2002, 251, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, D.; Ren, L.; Zhao, X.; Qin, J. Solid lipid nanoparticles for enhancing vinpocetine’s oral bioavailability. J. Control. Release 2006, 114, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Vyas, T.K.; Babbar, A.; Sharma, R.; Misra, A. Intranasal mucoadhesive microemulsions of zolmitriptan: Preliminary studies on brain-targeting. J. Drug Target. 2005, 13, 317–324. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, R.K.; Sharma, N.; Gabrani, R.; Sharma, S.K.; Ali, J.; Dang, S. Nose-to-brain delivery of PLGA-diazepam nanoparticles. AAPS PharmSciTech 2015, 16, 1108–1121. [Google Scholar] [CrossRef] [Green Version]
- Snehalatha, M.; Kolachina, V.; Saha, R.N.; Babbar, A.K.; Sharma, N.; Sharma, R.K. Enhanced tumor uptake, biodistribution and pharmacokinetics of etoposide loaded nanoparticles in Dalton’s lymphoma tumor bearing mice. J. Pharm. Bioallied Sci. 2013, 5, 290. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Neupane, Y.R.; Mangla, B.; Kohli, K. Nanostructured Lipid Carriers for Oral Bioavailability Enhancement of Exemestane: Formulation Design, In Vitro, Ex Vivo, and In Vivo Studies. J. Pharm. Sci. 2019, 108, 3382–3395. [Google Scholar] [CrossRef]
- Rajinikanth, P.S.; Chellian, J. Development and evaluation of nanostructured lipid carrier-based hydrogel for topical delivery of 5-fluorouracil. Int. J. Nanomed. 2016, 11, 5067. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hu, W.; Chen, H.; Ni, Q.; Xu, H.; Yang, X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int. J. Pharm. 2007, 328, 191–195. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.R.; Goyal, A.K. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 2016, 23, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Wang, P.-W.; Alalaiwe, A.; Lin, Z.-C.; Fang, J.-Y. Use of lipid Nanocarriers to improve Oral delivery of vitamins. Nutrients 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, H.A.; Javadzadeh, Y.; Hamidi, M.; Jalali, M.B. Repaglinide-loaded solid lipid nanoparticles: Effect of using different surfactants/stabilizers on physicochemical properties of nanoparticles. DARU J. Pharm. Sci. 2015, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Ghanbarzadeh, B.; Mohammadi, M.; Fathollahi, I.; Hamishehkar, H. Encapsulation of vitamin A palmitate in nanostructured lipid carrier (NLC)-effect of surfactant concentration on the formulation properties. Adv. Pharm. Bull. 2014, 4, 563. [Google Scholar] [PubMed]
- Das, S.; Ng, W.K.; Kanaujia, P.; Kim, S.; Tan, R.B. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: Effects of process variables. Colloids Surf. B Biointerfaces 2011, 88, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, H.; Sahito, B.; Li, X.; Peng, L.; Gao, X.; Ji, H.; Wang, L.; Jiang, S.; Guo, D. Nanostructured lipid carriers with exceptional gastrointestinal stability and inhibition of P-gp efflux for improved oral delivery of tilmicosin. Colloids Surf. B Biointerfaces 2019, 187, 110649. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrini, L.; Maestrelli, F.; Mennini, N.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Deliv. 2018, 25, 1910–1921. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 239–250. [Google Scholar]
- Lin, C.-Y.; Hsu, C.-Y.; Elzoghby, A.O.; Alalaiwe, A.; Hwang, T.-L.; Fang, J.-Y. Oleic acid as the active agent and lipid matrix in cilomilast-loaded nanocarriers to assist PDE4 inhibition of activated neutrophils for mitigating psoriasis-like lesions. Acta Biomater. 2019, 90, 350–361. [Google Scholar] [CrossRef]
- Son, G.-H.; Na, Y.-G.; Huh, H.W.; Wang, M.; Kim, M.-K.; Han, M.-G.; Byeon, J.-J.; Lee, H.-K.; Cho, C.-W. Systemic Design and Evaluation of Ticagrelor-Loaded Nanostructured Lipid Carriers for Enhancing Bioavailability and Antiplatelet Activity. Pharmaceutics 2019, 11, 222. [Google Scholar] [CrossRef] [Green Version]
- Chinsriwongkul, A.; Chareanputtakhun, P.; Ngawhirunpat, T.; Rojanarata, T.; Sila-on, W.; Ruktanonchai, U.; Opanasopit, P. Nanostructured lipid carriers (NLC) for parenteral delivery of an anticancer drug. AAPS PharmSciTech 2012, 13, 150–158. [Google Scholar] [CrossRef]
- Shen, J.; Sun, M.; Ping, Q.; Ying, Z.; Liu, W. Incorporation of liquid lipid in lipid nanoparticles for ocular drug delivery enhancement. Nanotechnology 2009, 21, 025101. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Zadeh, B.S.M.; Kazemi, M. Preparation and optimization of polymeric micelles as an oral drug delivery system for deferoxamine mesylate: In vitro and ex vivo studies. Res. Pharm. Sci. 2019, 14, 293. [Google Scholar]
- Khan, A.A.; Abdulbaqi, I.M.; Assi, R.A.; Murugaiyah, V.; Darwis, Y. Lyophilized hybrid nanostructured lipid carriers to enhance the cellular uptake of verapamil: Statistical optimization and in vitro evaluation. Nanoscale Res. Lett. 2018, 13, 323. [Google Scholar] [CrossRef] [PubMed]
- Jenning, V.; Thünemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Swidan, S.A.; Ghonaim, H.M.; Samy, A.M.; Ghorab, M.M. Efficacy and in vitro cytotoxicity of nanostructured lipid carriers for paclitaxel delivery. J. Appl. Pharm. Sci. 2016, 6, 018–026. [Google Scholar] [CrossRef] [Green Version]
- Lockman, P.; Koziara, J.; Mumper, R.; Allen, D. Nanoparticle Surface Charges Alter Blood–Brain Barrier Integrity and Permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef]
- Bramini, M.; Ye, D.; Hallerbach, A.; Raghnaill, M.; Salvati, A.; Aberg, C.; Dawson, K. Imaging Approach to Mechanistic Study of Nanoparticle Interactions with the Blood-Brain Barrier. ACS Nano 2014, 8. [Google Scholar] [CrossRef]
- Hanada, S.; Fujioka, K.; Inoue, Y.; Kanaya, F.; Manome, Y.; Yamamoto, K. Cell-based in vitro blood-brain barrier model can rapidly evaluate nanoparticles’ brain permeability in association with particle size and surface modification. Int. J. Mol. Sci. 2014, 15, 1812–1825. [Google Scholar] [CrossRef] [Green Version]
- Sanad, R.A.; AbdelMalak, N.S.; Badawi, A.A. Formulation of a novel oxybenzone-loaded nanostructured lipid carriers (NLCs). AAPS Pharmscitech 2010, 11, 1684–1694. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Liu, Z.; Lin, X.; He, H.; Tang, X. Formulation and characterization of bufadienolides-loaded nanostructured lipid carriers. Drug Dev. Ind. Pharm. 2010, 36, 508–517. [Google Scholar] [CrossRef]
- Sharma, P.; Ganta, S.; Denny, W.A.; Garg, S. Formulation and pharmacokinetics of lipid nanoparticles of a chemically sensitive nitrogen mustard derivative: Chlorambucil. Int. J. Pharm. 2009, 367, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Swidan, S.A.; Ghonaim, H.M.; Samy, A.M.; Ghorab, M.M. Comparative study of solid lipid nanoparticles and nanostructured lipid carriers for in vitro Paclitaxel delivery. J. Chem. Pharm. Res. 2016, 8, 482–493. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, E.L.; Berkó, S.; Gácsi, A.; Kovács, A.; Katona, G.; Soós, J.; Csányi, E.; Gróf, I.; Harazin, A.; Deli, M.A.; et al. Design and Optimization of Nanostructured Lipid Carrier Containing Dexamethasone for Ophthalmic Use. Pharmaceutics 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; Imam, S.S.; Aqil, M.; Ahad, A.; Sultana, Y.; Ameeduzzafar; Ali, A. Carvedilol nano lipid carriers: Formulation, characterization and in-vivo evaluation. Drug Deliv. 2016, 23, 1486–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saedi, A.; Rostamizadeh, K.; Parsa, M.; Dalali, N.; Ahmadi, N. Preparation and characterization of nanostructured lipid carriers as drug delivery system: Influence of liquid lipid types on loading and cytotoxicity. Chem. Phys. Lipids 2018, 216, 65–72. [Google Scholar] [CrossRef]
- Hamdani, J.; Moës, A.J.; Amighi, K. Physical and thermal characterisation of Precirol® and Compritol® as lipophilic glycerides used for the preparation of controlled-release matrix pellets. Int. J. Pharm. 2003, 260, 47–57. [Google Scholar] [CrossRef]
- Weiss, J.; Muschiolik, G. Factors Affecting the Droplet Size of Water-in-Oil Emulsions (W/O) and the Oil Globule Size in Water-in-Oil-in-Water Emulsions (W/O/W). J. Dispers. Sci. Technol. 2007, 28, 703–716. [Google Scholar] [CrossRef]
- Kamel, A.E.; Fadel, M.; Louis, D. Curcumin-loaded nanostructured lipid carriers prepared using Peceol™ and olive oil in photodynamic therapy: Development and application in breast cancer cell line. Int. J. Nanomed. 2019, 14, 5073–5085. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.H.; Ramasamy, T.; Truong, D.H.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS Pharmscitech 2014, 15, 1509–1515. [Google Scholar] [CrossRef] [Green Version]
- Teng, Z.; Yu, M.; Ding, Y.; Zhang, H.; Shen, Y.; Jiang, M.; Liu, P.; Opoku-Damoah, Y.; Webster, T.J.; Zhou, J. Preparation and characterization of nimodipine-loaded nanostructured lipid systems for enhanced solubility and bioavailability. Int. J. Nanomed. 2019, 14, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagoubi, A.S.; Shahidi, F.; Mohebbi, M.; Varidi, M.; Golmohammadzadeh, S. Preparation, characterization and evaluation of physicochemical properties of phycocyanin-loaded solid lipid nanoparticles and nanostructured lipid carriers. J. Food Meas. Charact. 2018, 12, 378–385. [Google Scholar] [CrossRef]
- Ana, R.; Mendes, M.; Sousa, J.; Pais, A.; Falcão, A.; Fortuna, A.; Vitorino, C. Rethinking carbamazepine oral delivery using polymer-lipid hybrid nanoparticles. Int. J. Pharm. 2019, 554, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Gönüllü, Ü.; Üner, M.; Yener, G.; Fatma Karaman, E.; Aydoğmuş, Z. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharm. 2015, 65, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Shah, F.A.; Rana, I.; Ansari, M.M.; ud Din, F.; Rizvi, S.Z.H.; Aman, W.; Lee, G.-Y.; Lee, E.-S.; Kim, J.-K. Nanostructured lipid carriers-mediated brain delivery of carbamazepine for improved in vivo anticonvulsant and anxiolytic activity. Int. J. Pharm. 2020, 119033. [Google Scholar] [CrossRef]
- Khan, A.A.; Mudassir, J.; Akhtar, S.; Murugaiyah, V.; Darwis, Y. Freeze-dried lopinavir-loaded nanostructured lipid carriers for enhanced cellular uptake and bioavailability: Statistical optimization, in vitro and in vivo evaluations. Pharmaceutics 2019, 11, 97. [Google Scholar] [CrossRef] [Green Version]
- Fini, A.; Cavallari, C.; Ospitali, F.; Gonzalez-Rodriguez, M.L. Theophylline-loaded compritol microspheres prepared by ultrasound-assisted atomization. J. Pharm. Sci. 2011, 100, 743–757. [Google Scholar] [CrossRef]
- Brubach, J.; Jannin, V.; Mahler, B.; Bourgaux, C.; Lessieur, P.; Roy, P.; Ollivon, M. Structural and thermal characterization of glyceryl behenate by X-ray diffraction coupled to differential calorimetry and infrared spectroscopy. Int. J. Pharm. 2007, 336, 248–256. [Google Scholar] [CrossRef]
- Souto, E.; Mehnert, W.; Müller, R. Polymorphic behaviour of Compritol® 888 ATO as bulk lipid and as SLN and NLC. J. Microencapsul. 2006, 23, 417–433. [Google Scholar] [CrossRef]
- Temazepam, C.; National Center for Biotechnology Information. PubChem Database. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Temazepam (accessed on 27 January 2020).
- Zeb, A.; Qureshi, O.S.; Kim, H.S.; Kim, M.S.; Kang, J.H.; Park, J.S.; Kim, J.K. High payload itraconazole-incorporated lipid nanoparticles with modulated release property for oral and parenteral administration. J. Pharm. Pharmacol. 2017, 69, 955–966. [Google Scholar] [CrossRef]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef]
- Ghazizadeh, F.; Ghaffari, S.; Mirshojaei, S.F.; Mazidid, M.; Azarmi, S. Biodistribution of Tc-99m Labeled Isoniazid Solid Lipid Nanoparticles in Wistar Rats. Iran. J. Pharm. Res. IJPR 2018, 17, 1209. [Google Scholar]
- Mohsin, K.; Alamri, R.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Hussain, M.D. Development of self-nanoemulsifying drug delivery systems for the enhancement of solubility and oral bioavailability of fenofibrate, a poorly water-soluble drug. Int. J. Nanomed. 2016, 11, 2829. [Google Scholar]
- Hoosain, F.G.; Choonara, Y.E.; Tomar, L.K.; Kumar, P.; Tyagi, C.; du Toit, L.C.; Pillay, V. Bypassing P-glycoprotein drug efflux mechanisms: Possible applications in pharmacoresistant schizophrenia therapy. BioMed Res. Int. 2015, 2015, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Zakeri-Milani, P.; Valizadeh, H. Intestinal transporters: Enhanced absorption through P-glycoprotein-related drug interactions. Expert Opin. Drug Metab. Toxicol. 2014, 10, 859–871. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Lima, S.A.C.; Figueiredo, F.; Fernandes, R.; Reis, S. Cellular uptake and transcytosis of lipid-based nanoparticles across the intestinal barrier: Relevance for oral drug delivery. J. Colloid Interface Sci. 2016, 463, 258–265. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012, 20, 813–830. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Zeb, A.; Cha, J.-H.; Noh, A.R.; Qureshi, O.S.; Kim, K.-W.; Choe, Y.-H.; Shin, D.; Shah, F.A.; Majid, A.; Bae, O.-N. Neuroprotective effects of carnosine-loaded elastic liposomes in cerebral ischemia rat model. J. Pharm. Investig. 2019, 49, 1–9. [Google Scholar] [CrossRef]












| Factors | Levels | Responses | Constrains | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Goal | ||||||
| A | 1 | 2 | 3 | 5 | R1 | 24.95 | 100.13 | Maximize |
| R2 | 254.6 | 1972.0 | Minimize | |||||
| B | Oleic | Labrasol® | Capryol® 90 | Miglyol® 840 | R3 | 0.017 | 1.00 | Minimize |
| R4 | −10.40 | 0.24 | Minimize | |||||
| Run | Stabilizer Conc. (%w/v) | Liquid Lipid Type | EE (%) (±SD) | ɀ Average d.nm (±SD) | PDI (±SD) | Zeta Potential (ZP) mV (±SD) |
|---|---|---|---|---|---|---|
| 1 | 1 | oleic | 75.2 ± 0.1 | 306.6 ± 49.6 | 0.09 ± 0.10 | −10.2 ± 0.3 |
| 2 | 2 | oleic | 99.8 ± 0.3 | 401.6 ± 51.8 | 0.21 ± 0.12 | −8.8 ± 0.3 |
| 3 | 3 | oleic | 75.6 ± 0.3 | 440.2 ± 36.8 | 0.76 ± 0.06 | −8.8 ± 0.3 |
| 4 | 5 | oleic | 81.3 ± 0.4 | 357.9 ± 43.1 | 0.65 ± 0.10 | −2.4 ± 0.2 |
| 5 | 1 | Labrasol® | 46.5 ± 0.1 | 477.8 ± 50.2 | 0.11 ± 0.09 | −3.6 ± 0.1 |
| 6 | 2 | Labrasol® | 56.5 ± 0.1 | 656.7 ± 105.8 | 0.93 ± 0.06 | 0.06 ± 0.19 |
| 7 | 3 | Labrasol® | 73.9 ± 0.2 | 695.8 ± 163.5 | 0.34 ± 0.33 | −1.1 ± 0.0 |
| 8 | 5 | Labrasol® | 86.9 ± 0.7 | 1658.7 ± 325.1 | 0.56 ± 0.46 | −0.19 ± 0.07 |
| 9 | 1 | Caproyl® 90 | 31.6 ± 0.1 | 512.7 ± 56.7 | 0.14 ± 0.11 | −5.2 ± 1.2 |
| 10 | 2 | Caproyl® 90 | 25.1 ± 0.1 | 489.2 ± 66.6 | 0.10 ± 0.09 | −0.08 ± 0.05 |
| 11 | 3 | Caproyl® 90 | 50.8 ± 0.1 | 708.9 ± 90.1 | 0.25 ± 0.14 | −0.31 ± 0.03 |
| 12 | 5 | Caproyl® 90 | 79.9 ± 0.4 | 1251.3 ± 228.8 | 0.09 ± 0.01 | −0.21 ± 0.02 |
| 13 | 1 | Miglyol® 840 | 34.5 ± 0.3 | 558.5 ± 42.1 | 0.69 ± 0.28 | −5.4 ± 0.5 |
| 14 | 2 | Miglyol® 840 | 52.0 ± 0.9 | 490.1 ± 76.8 | 0.85 ± 0.13 | −0.64 ± 0.59 |
| 15 | 3 | Miglyol® 840 | 61.9 ± 0.8 | 740.2 ± 111.7 | 0.46 ± 0.45 | −0.47 ± 0.06 |
| 16 | 5 | Miglyol® 840 | 98.4 ± 0.8 | 1421.7 ± 376.2 | 1 ± 0 | −0.80 ± 0.11 |
| Parameters | SS | DF | MS | F | p-Value | Parameters | SS | DF | MS | F | p-Value |
| Model | EE% | Model | PDI | ||||||||
| Cubic | 22266.26 | 9 | 2474.03 | 50.3 | <0.0001 | Cubic | 2.36 | 9 | 0.26 | 5.04 | 0.0002 |
| A | 351.4 | 1 | 351.4 | 7.2 | 0.0111 | A | 0.06 | 1 | 0.06 | 1.21 | 0.2793 |
| B | 1981.7 | 1 | 1981.7 | 40.3 | <0.0001 | B | 0.67 | 1 | 0.67 | 12.79 | 0.0010 |
| AB | 3294.4 | 1 | 3294.4 | 67.0 | <0.0001 | AB | 0.22 | 1 | 0.22 | 4.30 | 0.0451 |
| A2 | 12.2 | 1 | 12.2 | 0.25 | 0.6220 | A2 | 0.02 | 1 | 0.02 | 0.44 | 0.5092 |
| B2 | 2570.3 | 1 | 2570.3 | 52.3 | <0.0001 | B2 | 0.68 | 1 | 0.68 | 13.01 | 0.0009 |
| A2B | 217.1 | 1 | 217.1 | 4.4 | 0.0425 | A2B | 0.28 | 1 | 0.28 | 5.34 | 0.0266 |
| AB2 | 493.6 | 1 | 493.6 | 10.0 | 0.0031 | AB2 | 0.10 | 1 | 0.10 | 1.98 | 0.1673 |
| A3 | 40.9 | 1 | 40.9 | 0.83 | 0.3678 | A3 | 0.09 | 1 | 0.09 | 1.73 | 0.1971 |
| B3 | 781.9 | 1 | 781.9 | 15.9 | 0.0003 | B3 | 0.62 | 1 | 0.62 | 11.83 | 0.0015 |
| Parameters | SS | DF | MS | F | p-Value | Parameters | SS | DF | MS | F | p-Value |
| Model | Particle Size | Model | Zeta Potential | ||||||||
| Cubic | 3.6 × 10−3 | 9 | 4.0 × 10−4 | 30.1 | <0.0001 | Cubic | 558.7 | 9 | 62.1 | 121.7 | <0.0001 |
| A | 1.7 × 10−4 | 1 | 1.7 × 10−4 | 12.7 | 0.0010 | A | 9.1 | 1 | 9.1 | 17.8 | 0.0002 |
| B | 8.7 × 10−5 | 1 | 8.7 × 10−5 | 6.5 | 0.0152 | B | 0.67 | 1 | 0.67 | 1.3 | 0.2578 |
| AB | 1 × 10−4 | 1 | 1 × 10−4 | 7.35 | 0.0101 | AB | 5.4 | 1 | 5.4 | 10.5 | 0.0025 |
| A2 | 2.9 × 10−7 | 1 | 2.9 × 10−7 | 0.02 | 0.8822 | A2 | 5.3 | 1 | 5.3 | 10.5 | 0.0026 |
| B2 | 6.6 × 10−4 | 1 | 6.6 × 10−4 | 49.1 | <0.0001 | B2 | 118.9 | 1 | 118.9 | 233.2 | <0.0001 |
| A2B | 1.6 × 10−4 | 1 | 1.6 × 10−4 | 11.9 | 0.0014 | A2B | 35.9 | 1 | 35.9 | 70.4 | <0.0001 |
| AB2 | 1.2 × 10−4 | 1 | 1.2 × 10−4 | 8.8 | 0.0052 | AB2 | 9.7 | 1 | 9.7 | 18.9 | 0.0001 |
| A3 | 1.1 × 10−5 | 1 | 1.1 × 10−5 | 0.81 | 0.3726 | A3 | 23.2 | 1 | 23.2 | 45.6 | <0.0001 |
| B3 | 2.4 × 10−4 | 1 | 2.4 × 10−4 | 17.6 | 0.0002 | B3 | 25.2 | 1 | 25.2 | 49.3 | <0.0001 |
| Liquid Lipid Type | General Class | Chemical Name | C-Length | M.wt Average (g/mol) | Viscosity (20 °C) (mPa·s) | Hydrophilic-Lipophilic Balance (HLB) |
|---|---|---|---|---|---|---|
| Oleic acid | Fatty acids | Octadec-9-enoic acid | C18 | 282.5 | 40 | 1 |
| Labrasol® | Polyglycolyzed glycerides | Caprylo caproyl Polyoxyl-8 glycerides | C8–C10 | 400 | 80–110 | 12 |
| Capryol® 90 | PG fatty acid esters | Propylene glycol monocaprylate (type II) | C8 | 202.29 | 20 | 5 |
| Miglyol® 840 | PG fatty acid esters | Propylene glycol diester of saturated vegetable fatty acids | C8-C10 | 709 | 9–12 | <10 |
| Formulation | Organ | Time Points (h) | ||||
|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 2 h | 4h | 6 h | ||
| 99mTc-temazepam suspension | Blood | 0.022 ± 0.005 | 0.036 ± 0.004 | 0.032 ± 0.002 | 0.021 ± 0.001 | 0.013 ± 0.001 |
| 99mTc-temazepam NLC-1 | Blood | 0.036 ± 0.004 | 0.050 ± 0.003 | 0.043 ± 0.005 | 0.040 ± 0.006 | 0.035 ± 0.007 |
| 99mTc-temazepam suspension | Brain | 0.020 ± 0.007 | 0.025 ± 0.004 | 0.035 ± 0.003 | 0.019 ± 0.004 | 0.012 ± 0.004 |
| 99mTc-temazepam NLC-1 | Brain | 0.040 ± 0.002 | 0.070 ± 0.003 | 0.053 ± 0.007 | 0.045 ± 0.005 | 0.040 ± 0.004 |
| 99mTc-temazepam suspension | Brain/blood | 0.90 | 0.69 | 1.09 | 0.90 | 0.92 |
| 99mTc-temazepam NLC-1 | Brain/blood | 1.11 | 1.40 | 1.23 | 1.13 | 1.14 |
| Formulation | Organ | Cmax (%id/g) | Tmax | AUC0–t | AUC0–∞ | MRT0-inf_obs (h) | Cl/F_obs (µci/%id/g/h) | t1/2 (h) |
|---|---|---|---|---|---|---|---|---|
| (h) | (%id/g*h) | (%id/g*h) | ||||||
| 99mTc-temazepam suspension | Blood | 0.039 ± 0.001 | 1.3 ± 0.5 | 0.14 ± 0.02 | 0.23 ± 0.11 | 4.1 ± 0.4 | 467.5 ± 21.7 | 2.5 ± 0.4 |
| 99mTc-temazepam NLC-1 | Blood | 0.057 * ± 0.012 | 1.3 ns ± 0.6 | 0.23 * ± 0.04 | 0.68 * ± 0.25 | 13.4 * ± 5.2 | 145.8 **** ± 16.4 | 8.9 * ± 3.5 |
| 99mTc-temazepam suspension | Brain | 0.033 ± 0.006 | 2.0 ± 0.2 | 0.13 ± 0.03 | 0.19 ± 0.08 | 4.9 ± 1.5 | 532.8 ± 52.7 | 2.3 ± 0.3 |
| 99mTc-temazepam NLC-1 | Brain | 0.070 *** ± 0.001 | 1.0 *** ± 0.1 | 0.27 ** ± 0.04 | 0.65 *** ± 0.01 | 10.8 * ± 2.5 | 119.9 **** ± 1.9 | 7.3 * ± 2.0 |
| Re | 3.38 | |||||||
| Ce | 2.12 | |||||||
| Drug-targeting index (DTI) | 1.16 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
E. Eleraky, N.; M. Omar, M.; A. Mahmoud, H.; A. Abou-Taleb, H. Nanostructured Lipid Carriers to Mediate Brain Delivery of Temazepam: Design and In Vivo Study. Pharmaceutics 2020, 12, 451. https://doi.org/10.3390/pharmaceutics12050451
E. Eleraky N, M. Omar M, A. Mahmoud H, A. Abou-Taleb H. Nanostructured Lipid Carriers to Mediate Brain Delivery of Temazepam: Design and In Vivo Study. Pharmaceutics. 2020; 12(5):451. https://doi.org/10.3390/pharmaceutics12050451
Chicago/Turabian StyleE. Eleraky, Nermin, Mahmoud M. Omar, Hemat A. Mahmoud, and Heba A. Abou-Taleb. 2020. "Nanostructured Lipid Carriers to Mediate Brain Delivery of Temazepam: Design and In Vivo Study" Pharmaceutics 12, no. 5: 451. https://doi.org/10.3390/pharmaceutics12050451