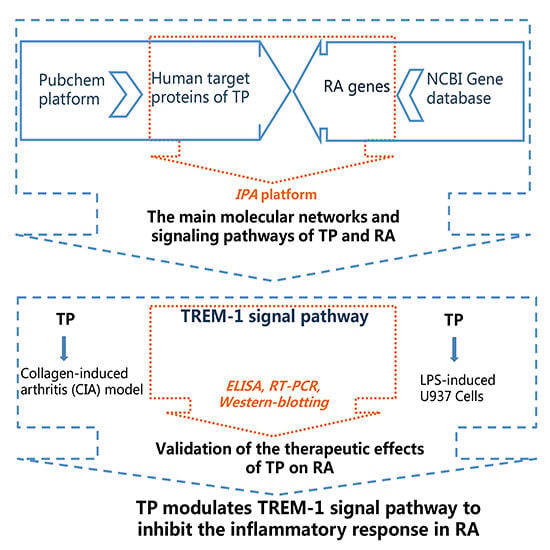
Triptolide Modulates TREM-1 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Triggering Receptors Expressed on Myeloid Cells (TREM)-1 Signaling Was an Important Signaling Pathway of Triptolide (TP) on Inflammatory Response in Rheumatoid Arthritis (RA)
2.1.2. TP Inhibited the Activation of TREM-1 Signal Pathway and Production of Inflammatory Cytokines in Lipopolysaccharides (LPS)-Induced U937 Cells
2.1.3. TREM-1 Signal Pathway Might Participate in the Regulatory Effect of TP on LPS-Induced Inflammatory Cytokines Production
2.1.4. TP Alleviated the Severity of Arthritis
2.1.5. TP Suppressed TREM-1 Signal Pathway in Ankle Joints of Collagen-Induced Arthritis (CIA) Rats
2.1.6. TP Reduced the Expression of Proinflammatory Cytokines in Serum and Ankle Joints of CIA Rats
2.2. Discussion
3. Experimental Section
3.1. Analysis of Molecular Networks and Signaling Pathways of TP and RA
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Treatment of U937 Cells with TP
3.5. siRNA Transfection
3.6. Animals and Experimental Protocol
3.6.1. Animals
3.6.2. Induction of CIA and Assessment of Arthritis
3.6.3. Treatment
3.6.4. Flow Cytometry
3.6.5. ELISA Analysis
3.6.6. Real-Time PCR
3.6.7. Western Blot Analysis
3.6.8. Histological Assay
3.7. Statistic Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Onozaki, K. Etiological and biological aspects of cigarette smoking in rheumatoid arthritis. Inflamm. Allergy Drug Targets 2009, 8, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Zwerina, J.; Redlich, K.; Schett, G.; Smolen, J.S. Pathogenesis of rheumatoid arthritis: Targeting cytokines. Ann. N. Y. Acad. Sci. 2005, 1051, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Stuhlmuller, B.; Keyszer, G.; Kinne, R.W. Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum. 1997, 40, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Strieter, R.M.; Kunkel, S.L.; Koch, A.E. Chemokines in rheumatoid arthritis. Springer Semin. Immunopathol. 1998, 20, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.U.; Kwok, S.K.; Hong, K.H.; Yoo, S.A.; Kong, J.S.; Choe, J.; Cho, C.S. Soluble Fas ligand inhibits angiogenesis in rheumatoid arthritis. Arthritis Res. Ther. 2007, 9. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, S.P.; Thumboo, J.; Vasoo, S.; Thio, S.T.; Tse, C.; Fong, K.Y. In vivo pro- and anti-inflammatory cytokines in normal and patients with rheumatoid arthritis. Ann. Acad. Med. Singap. 2007, 36, 96–99. [Google Scholar] [PubMed]
- Koch, A.E. Angiogenesis as a target in rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62 (Suppl. 2), ii60–ii67. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.I.; Cusick, M.E.; Valle, D.; Childs, B.; Vidal, M.; Barabasi, A.L. The human disease network. Proc. Natl. Acad. Sci. USA 2007, 104, 8685–8690. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhou, J.; He, Y.; Jia, H.; Zhao, L.; Zhao, N.; Lu, A. Effects of Triptolide from Radix Tripterygium wilfordii (Leigongteng) on cartilage cytokines and transcription factor NF-κB: A study on induced arthritis in rats. Chin. Med. 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Tengchaisri, T.; Chawengkirttikul, R.; Rachaphaew, N.; Reutrakul, V.; Sangsuwan, R.; Sirisinha, S. Antitumor activity of Triptolide against cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett. 1998, 133, 169–175. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, J.; Bao, X.; Chan, S.; Young, D.O.; Liu, D.; Shen, P. Triptolide attenuates oxidative stress, NF-κB activation and multiple cytokine gene expression in murine peritoneal macrophage. Int. J. Mol. Med. 2006, 17, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Cush, J.J.; Garret, M.; Lipsky, P.E. A phase I study of ethyl acetate extract of the Chinese antirheumatic herb Tripterygium wilfordii Hook F in rheumatoid arthritis. J. Rheumatol. 2001, 28, 2160–2167. [Google Scholar] [PubMed]
- Calvano, S.E.; Xiao, W.; Richards, D.R.; Felciano, R.M.; Baker, H.V.; Cho, R.J.; Chen, R.O.; Brownstein, B.H.; Cobb, J.P.; Tschoeke, S.K.; et al. A network-based analysis of systemic inflammation in humans. Nature 2005, 437, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Davis, L.S.; Lipsky, P.E. Effect of an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F on human immune responsiveness. Arthritis Rheum. 1991, 34, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.Z.; Brandwein, S.R.; Banerjee, S. Inhibition of type II collagen induced arthritis in mice by an immunosuppressive extract of Tripterygium wilfordii Hook F. J. Rheumatol. 1992, 19, 682–688. [Google Scholar] [PubMed]
- Kupchan, S.M.; Court, W.A.; Dailey, R.G., Jr.; Gilmore, C.J.; Bryan, R.F. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J. Am. Chem. Soc. 1972, 94, 7194–7195. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Matsuishi, J.; Yu, Y.; Kasahara, T.; Hisamitsu, T. Suppressive effects of Tripterygium wilfordii Hook F., a traditional Chinese medicine, on collagen arthritis in mice. Immunopharmacology 1998, 39, 117–126. [Google Scholar] [CrossRef]
- Wang, B.; Ma, L.; Tao, X.; Lipsky, P.E. Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum. 2004, 50, 2995–2303. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.T.; Feng, L.; Yao, H.P.; Sun, W.J.; Zhang, L.H. Effect of Triptolide on TNFα-induced activation of NF-κB and expression of COX-2 and iNOS in human rheumatoid arthritis synovial fibroblasts. Zhejiang Da Xue Xue Bao. Yi Xue Ban = J. Zhejiang Univ. Med. Sci. 2004, 33, 160–165. [Google Scholar]
- Zhang, N.; Xu, Y.J.; Zhang, Z.X. Regulatory function of nuclear factor κ B on lymphocyte proliferation and apoptosis in bronchial asthmatic rats and effect of Triptolide on the regulation. Zhongguo Zhong Xi Yi Jie He Za Zhi Chin. 2004, 24, 435–438. [Google Scholar]
- Geng, Y.; Fang, M.; Wang, J.; Yu, H.; Hu, Z.; Yew, D.T.; Chen, W. Triptolide down-regulates COX-2 expression and PGE2 release by suppressing the activity of NF-κB and MAP kinases in lipopolysaccharide-treated PC12 cells. Phytother. Res. PTR 2012, 26, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, V.; Dey, M.; Dorn, R.; Raskin, I. MyD88-dependent and independent pathways of Toll-like receptors are engaged in biological activity of Triptolide in ligand-stimulated macrophages. BMC Chem. Biol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Ornatowska, M.; Azim, A.C.; Wang, X.; Christman, J.W.; Xiao, L.; Joo, M.; Sadikot, R.T. Functional genomics of silencing TREM-1 on TLR4 signaling in macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L1377–L1384. [Google Scholar] [CrossRef] [PubMed]
- Rückert, R.; Brandt, K.; Ernst, M.; Marienfeld, K.; Csernok, E.; Metzler, C.; Budagian, V.; Bulanova, E.; Paus, R.; Bulfone-Paus, S. Interleukin-15 stimulates macrophages to activate CD4+ T cells: A role in the pathogenesis of rheumatoid arthritis? Immunology 2009, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, N.N.; Zhou, X.W.; Miao, P.; Hu, C.Y.; Qian, L.; Yu, Q.W.; Zhang, J.Y.; Nie, H.; Chen, X.; et al. Exogenous IFN-β regulates the RANKL-c-Fos-IFN-β signaling pathway in the collagen antibody-induced arthritis model. J. Transl. Med. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G. TREM-1: Intracellular signaling pathways and interaction with pattern recognition receptors. J. Leukoc. Biol. 2013, 93, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Tessarz, A.S.; Cerwenka, A. The TREM-1/DAP12 pathway. Immunol. Lett. 2008, 116, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Bleharski, J.R.; Kiessler, V.; Buonsanti, C.; Sieling, P.A.; Stenger, S.; Colonna, M.; Modlin, R.L. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 2003, 170, 3812–3818. [Google Scholar] [CrossRef] [PubMed]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef] [PubMed]
- Schenk, M.; Bouchon, A.; Seibold, F.; Mueller, C. TREM-1—Expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J. Clin. Investig. 2007, 117, 3097–3106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.T.; Kang, E.J.; Ha, Y.J.; Song, J.S. Levels of plasma-soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) are correlated with disease activity in rheumatoid arthritis. J. Rheumatol. 2012, 39, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.F.; Lesur, O.; Fulop, T., Jr. Effects of TREM-1 activation in human neutrophils: Activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int. Immunol. 2007, 19, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kuai, J.; Gregory, B.; Hill, A.; Pittman, D.D.; Feldman, J.L.; Brown, T.; Carito, B.; O’Toole, M.; Ramsey, R.; Adolfsson, O.; et al. TREM-1 expression is increased in the synovium of rheumatoid arthritis patients and induces the expression of pro-inflammatory cytokines. Rheumatology 2009, 48, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; La, D.T.; Yang, H.T.; Massin, F.; Gibot, S.; Faure, G.; Stohl, W. Elevated synovial expression of triggering receptor expressed on myeloid cells 1 in patients with septic arthritis or rheumatoid arthritis. Ann. Rheum. Dis. 2009, 68, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E., Jr. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, R.G.; Waldman, A.; Zhang, G.; Mitchell, A.; Tedla, N.; Cai, H.; Geczy, C.R.; Chesterman, C.N.; Perry, M.; Khachigian, L.M. Suppression of vascular permeability and inflammation by targeting of the transcription factor c-Jun. Nat. Biotechnol. 2006, 24, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Kano, A.; Wolfgang, M.J.; Gao, Q.; Jacoby, J.; Chai, G.X.; Hansen, W.; Iwamoto, Y.; Pober, J.S.; Flavell, R.A.; Fu, X.Y. Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. J. Exp. Med. 2003, 198, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Clausen, B.E.; Kaisho, T.; Tsujimura, T.; Terada, N.; Forster, I.; Akira, S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of STAT3 in macrophages and neutrophils. Immunity 1999, 10, 39–49. [Google Scholar] [CrossRef]
- Holmdahl, R.; Rubin, K.; Klareskog, L.; Dencker, L.; Gustafson, G.; Larsson, E. Appearance of different lymphoid cells in synovial tissue and in peripheral blood during the course of collagen II-induced arthritis in rats. Scand. J. Immunol. 1985, 21, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, R.; Jansson, L.; Andersson, M.; Larsson, E. Immunogenetics of type II collagen autoimmunity and susceptibility to collagen arthritis. Immunology 1988, 65, 305–310. [Google Scholar] [PubMed]
- Brenner, M.; Meng, H.C.; Yarlett, N.C.; Griffiths, M.M.; Remmers, E.F.; Wilder, R.L.; Gulko, P.S. The non-major histocompatibility complex quantitative trait locus CIA10 contains a major arthritis gene and regulates disease severity, pannus formation, and joint damage. Arthritis Rheum. 2005, 52, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Bendele, A.; McAbee, T.; Sennello, G.; Frazier, J.; Chlipala, E.; McCabe, D. Efficacy of sustained blood levels of interleukin-1 receptor antagonist in animal models of arthritis: Comparison of efficacy in animal models with human clinical data. Arthritis Rheum. 1999, 42, 498–506. [Google Scholar] [CrossRef]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, D.; He, X.; Bian, Y.; Guo, Q.; Zheng, K.; Zhao, Y.; Lu, C.; Liu, B.; Xu, X.; Zhang, G.; et al. Triptolide Modulates TREM-1 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis. Int. J. Mol. Sci. 2016, 17, 498. https://doi.org/10.3390/ijms17040498
Fan D, He X, Bian Y, Guo Q, Zheng K, Zhao Y, Lu C, Liu B, Xu X, Zhang G, et al. Triptolide Modulates TREM-1 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis. International Journal of Molecular Sciences. 2016; 17(4):498. https://doi.org/10.3390/ijms17040498
Chicago/Turabian StyleFan, Danping, Xiaojuan He, Yanqin Bian, Qingqing Guo, Kang Zheng, Yukun Zhao, Cheng Lu, Baoqin Liu, Xuegong Xu, Ge Zhang, and et al. 2016. "Triptolide Modulates TREM-1 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis" International Journal of Molecular Sciences 17, no. 4: 498. https://doi.org/10.3390/ijms17040498