- UMR BIOGER, Institut National De La Recherche Agronomique, AgroParisTech, Université Paris Saclay, Thiverval-Grignon, France
During infection, pathogens secrete an arsenal of molecules, collectively called effectors, key elements of pathogenesis which modulate innate immunity of the plant and facilitate infection. Some of these effectors can be recognized directly or indirectly by resistance (R) proteins from the plant and are then called avirulence (AVR) proteins. This recognition usually triggers defense responses including the hypersensitive response and results in resistance of the plant. R—AVR gene interactions are frequently exploited in the field to control diseases. Recently, the availability of fungal genomes has accelerated the identification of AVR genes in plant pathogenic fungi, including in fungi infecting agronomically important crops. While single AVR genes recognized by their corresponding R gene were identified, more and more complex interactions between AVR and R genes are reported (e.g., AVR genes recognized by several R genes, R genes recognizing several AVR genes in distinct organisms, one AVR gene suppressing recognition of another AVR gene by its corresponding R gene, two cooperating R genes both necessary to recognize an AVR gene). These complex interactions were particularly reported in pathosystems showing a long co-evolution with their host plant but could also result from the way agronomic crops were obtained and improved (e.g., through interspecific hybridization or introgression of resistance genes from wild related species into cultivated crops). In this review, we describe some complex R—AVR interactions between plants and fungi that were recently reported and discuss their implications for AVR gene evolution and R gene management.
Introduction
During infection, pathogens secrete an arsenal of molecules, collectively called effectors, key elements of pathogenesis which modulate innate immunity of the plant and facilitate infection (Oliva et al., 2010). Plants have evolved resistance (R) genes encoding R proteins able to recognize, directly or indirectly, some of these effectors [then called avirulence (AVR) proteins]. Recognition of a pathogen AVR protein triggers a set of immune responses grouped under the term Effector-Triggered Immunity (ETI), frequently leading to a rapid localized cell death termed the hypersensitive response (HR) (Jones and Dangl, 2006). Under the selection pressure exerted by R genes, pathogens can become virulent through evolution of their AVR gene repertoire. Mechanisms leading to virulence include complete deletion, inactivation, or down-regulation of the AVR gene, or point mutations allowing recognition to be evaded while maintaining the virulence function of the AVR protein (Jones and Dangl, 2006; Guttman et al., 2014). One class of R proteins corresponds to cell surface LRR-containing R proteins that are anchored to the plasma membrane via a transmembrane (TM) domain and sometimes include an intracellular kinase domain (Receptor-Like Proteins, RLP/Receptor like Kinases, RLK; Yang et al., 2012). The major class of identified R proteins however corresponds to intracellular nucleotide-binding and leucine-rich repeat receptors (NLR). NLR are multi-domain proteins containing a C-terminal leucine-rich repeat (LRR) domain, a central nucleotide-binding (NB) domain and a N-terminal domain often composed of a Toll/interleukin-1 receptor (TIR) or a coiled-coil (CC) domain (Takken and Goverse, 2012). Their multi-domain structure allows R proteins to simultaneously recognize AVR proteins and trigger plant defense reactions. Four models of AVR recognition by R proteins have been proposed and found to co-exist. In the elicitor-receptor model, the R protein directly recognizes its corresponding AVR protein and triggers defense responses (Keen, 1990; Jia et al., 2000; Dodds et al., 2006; Catanzariti et al., 2010; Steinbrenner et al., 2015). In the guard model, the interaction between R and AVR proteins is indirect: the R protein detects modifications of an effector's host target protein, called a “guardee” (Dangl and Jones, 2001). In the decoy model, the R protein detects modifications in a plant protein (called a “decoy”) that mimics the effector target and “traps” the AVR protein (van der Hoorn and Kamoun, 2008). Finally, in the recently proposed integrated decoy model, non-canonical domains mimicking the effector target are integrated into NLRs and play the role of “decoy” (Cesari et al., 2014a; Le Roux et al., 2015, Sarris et al., 2015).
Fungi are the most devastating pathogens of plants, including crops of major economic importance (Fisher et al., 2012). Genetic control is widely used to limit disease development, mainly through the use of major plant R genes recognizing fungal AVR genes. However, as more and more R and AVR genes are cloned and their molecular interactions are characterized, an increasing number of complex R—AVR gene interactions have been identified (Table 1). Such complex R—AVR gene interactions potentially result from long co-evolution between plants and pathogens and also from the way agronomic crops were obtained and improved, e.g., through interspecific hybridization or introgression of R genes from wild related species. In this review, we highlight some complex R—AVR gene interactions and discuss how they allow plants to expand pathogen recognition, how pathogens circumvent those plant resistances, and how complex interactions could be managed to improve crop disease resistance.
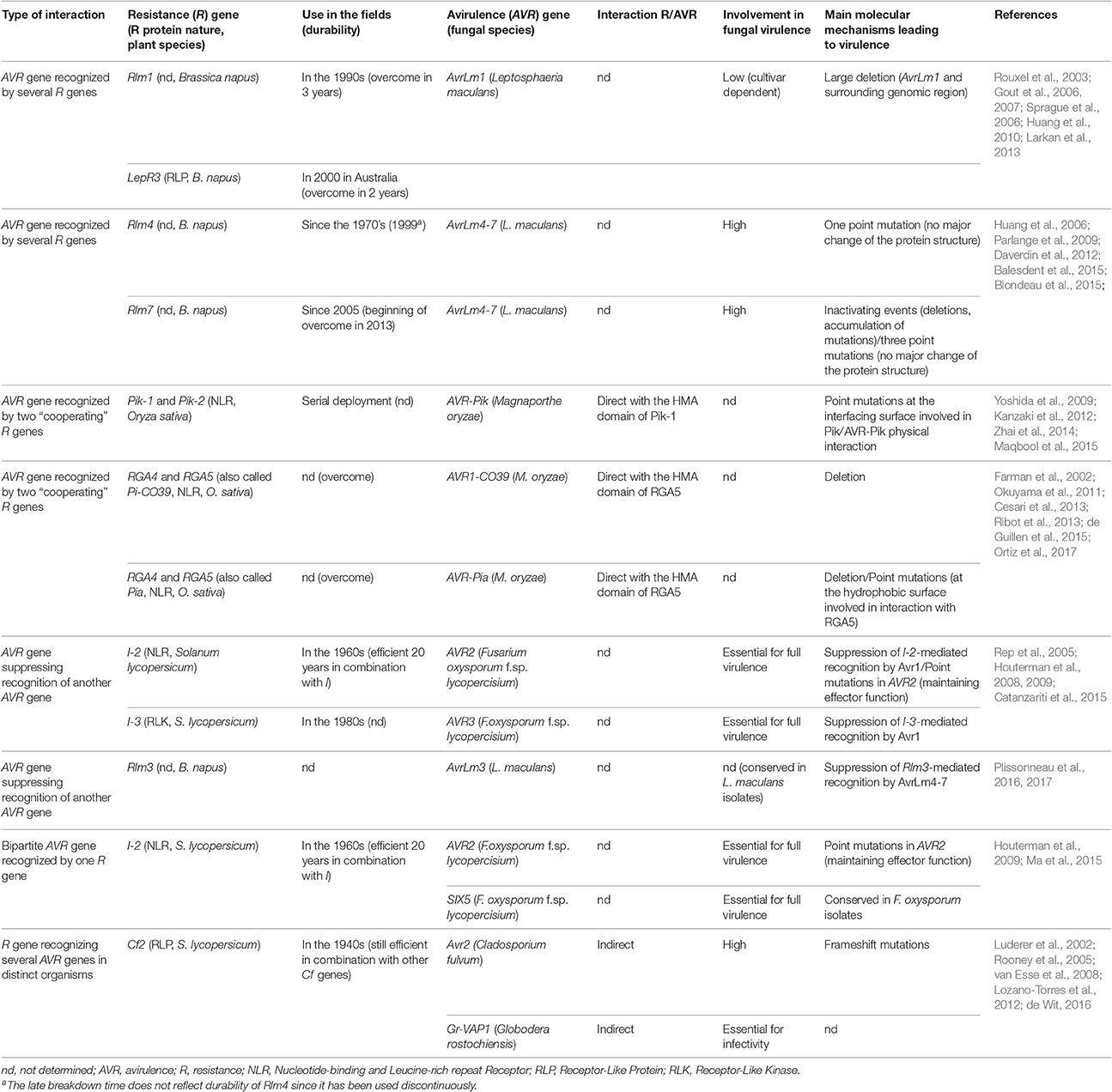
Table 1. Characteristics of fungal avirulence genes and plant resistance genes involved in complex interactions.
Avirulence Genes Recognized by Several Resistance Genes
AVR genes recognized by several R genes were reported in the pathosystem Leptosphaeria maculans/oilseed rape. L. maculans is a hemibiotrophic ascomycete responsible for stem canker (Blackleg) of oilseed rape (Brassica napus) and is mainly controlled using specific R genes often combined with quantitative resistance. To date, 7 AVR genes from L. maculans have been cloned and all are located in repeat-rich, gene-poor genomic regions (Rouxel and Balesdent, 2017).
AvrLm1 is recognized by two R genes, Rlm1 and LepR3. The two R genes are located on different chromosomes and are thus expected to encode different R proteins, although direct evidence is missing to date since only LepR3 has been cloned (through map-based cloning; Larkan et al., 2013). AvrLm1 is located as a solo gene in the middle of a 269 kb repeat-rich region. Rlm1 resistance was deployed in the 1990s and overcome in only 3 years (Rouxel et al., 2003). The main mechanism leading to virulence toward Rlm1 was a large deletion of AvrLm1 and its surrounding region (Gout et al., 2007), supporting a limited role of AvrLm1 in fungal fitness which is cultivar-dependent (Huang et al., 2010). More recently, AvrLm1 was reported to be recognized by the R protein LepR3, a RLP (Larkan et al., 2013). LepR3 resistance was rapidly overcome in parts of Australia soon after its introduction (Sprague et al., 2006) as a consequence of the previous use of Rlm1 cultivars and the deletion of AvrLm1 in a high proportion of Australian L. maculans isolates (Gout et al., 2007).
AvrLm4-7 is also recognized by two R genes, namely Rlm4 and Rlm7. It is unclear whether Rlm4 and Rlm7, which are clustered in the same linkage group but not cloned, are two different genes or two alleles of the same gene (Delourme et al., 2004). In the field, Rlm4 resistance has been extensively used since the 1970s but is now largely overcome (Rouxel and Balesdent, 2017). The switch to virulence against Rlm4 was due to a single non-synonymous mutation which does not modify the overall 3-D structure of AvrLm4-7 (Blondeau et al., 2015) and does not affect recognition by Rlm7 (Parlange et al., 2009). Rlm7 resistance was deployed in 2004 and then used extensively (e.g., Rlm7 cultivars comprised 50–70% of the French oilseed crop in 2013; Balesdent et al., 2015). However, the evolution of French L. maculans populations toward virulence against Rlm7 was a long process (4% of virulent isolates in 2010, 19% in 2013). The first molecular events leading to virulence toward Rlm7 mainly corresponded to drastic events (deletion, accumulation of mutations) and also to three amino acid changes without major modification of protein structure (Daverdin et al., 2012; Blondeau et al., 2015). The durability of Rlm7 resistance may reflect the importance of AvrLm4-7 for fungal fitness and aggressiveness (Huang et al., 2006) but also the introduction of Rlm7 into cultivars with high levels of quantitative resistance (Balesdent et al., 2015) and the antagonistic role of AvrLm4-7 on the AvrLm3/Rlm3 interaction (see section An avirulence gene suppressing recognition of another avirulence gene below). In contrast to the AvrLm1/Rlm1–LepR3 interaction, the AvrLm4-7/Rlm4–Rlm7 interaction illustrates that two R genes, or possibly two alleles of the same gene, targeting the same AVR gene can be deployed successively and be both durable in the field.
Avirulence Genes Recognized by Two “Cooperating” Resistance Genes
AVR genes recognized by two distinct R genes that are both necessary for recognition were reported in the Magnaporthe oryzae/rice pathosystem. M. oryzae, the causal agent of rice blast, is mostly controlled using resistant rice cultivars harboring major R genes. Seven M. oryzae AVR genes have been cloned (Liu et al., 2013). Interestingly, four of those AVR genes (AVR-Pik, AVR-Pii, AVR1-CO39, and AVR-Pia) are involved in complex interactions, in that two “cooperating” R genes are necessary to recognize each AVR (respectively Pik-1/Pik-2, Pii-1/Pii-2, and RGA4/RGA5; Okuyama et al., 2011; Kanzaki et al., 2012; Cesari et al., 2013; Takagi et al., 2013).
Okuyama et al. (2011) showed that AVR-Pia is recognized by two head-to-head R genes, RGA4 and RGA5, both being required for resistance. These R genes also recognize another M. oryzae AVR gene, AVR1-CO39 (Cesari et al., 2013). In this pair of R proteins, RGA4 acts as constitutively active disease resistance and cell death inducer and is repressed by RGA5 in absence of the pathogen. Direct binding of AVR-Pia or AVR1-CO39 to RGA5 leads to RGA4 de-repression and activation of immune signal transduction (Cesari et al., 2014b). Effector binding to RGA5 occurs in a non-canonical C-terminal domain of RGA5 (called the RATX1/HMA domain) resembling a heavy metal-associated (HMA) domain protein from Saccharomyces cerevisiae, thought to function as an integrated decoy domain (Cesari et al., 2013, 2014b; Kroj et al., 2016). The Pik locus is also composed of two head-to-head genes separated by a non-coding intergenic region and a HMA domain is present in Pik-1, in this case between the CC and NB domains (Yoshida et al., 2009; Kanzaki et al., 2012). A physical interaction has been demonstrated between AVR-Pik and the HMA domain of Pik-1 (Zhai et al., 2014). Both AVR-Pik and the HMA domain of Pik-1 exhibit amino acid polymorphisms between pathogen isolates and rice cultivars (Yoshida et al., 2009; Kanzaki et al., 2012), located at the interface between Pik-1 and AVR-Pik, meditating their physical interaction and recognition (Maqbool et al., 2015). In M. oryzae isolate collections, most are virulent toward Pia and Pi-CO39 and have lost AVR-Pia and AVR1-CO39 (Farman et al., 2002; Cesari et al., 2013). Three isolates virulent toward Pia were found to carry an AVR-Pia allele with a SNP leading to a non-synonymous substitution, which abolishes interaction with RGA5 and subsequent recognition (Cesari et al., 2013). Recently, Ortiz et al. (2017) found that binding of AVR-Pia to the RATX1 domain of RGA5 involved hydrophobic interactions and that AVR-Pia also interacted with other, as yet undefined, regions of RGA5, increasing the overall effector binding affinity of RGA5 and allowing AVR-Pia recognition and plant defense induction despite the accumulation of point mutations in Avr-Pia and moderate affinity to RATX1. This work highlights the advantage of integrating the decoy domain into the NLR, instead of having the decoy as an independent molecule. Indeed, even if physical interactions between R and AVR proteins favor diversification at the interfacing surfaces, the high resilience of RGA4/RGA5-mediated AVR-Pia recognition to reduction of AVR-Pia-RATX1 interaction strength limits the pathogen's ability to circumvent host recognition. The next step forward would be to fuse other effector targets to NLRs as integrated domains to test whether this can confer increased recognition specificity. These effector targets could themselves be engineered in order to be targeted by a larger panel of effectors and pathogens, such as PBS1 from A. thaliana, which cleavage by the bacterial protease AvrPphB is detected by the R protein RPS5, and in which substitution of AvrPphB cleavage site with cleavage sites from other effector proteases extended the recognition specificity of RPS5 to other pathogens (Kim et al., 2016).
An Avirulence Gene Suppressing Recognition of Another Avirulence Gene
Among the proposed roles of pathogen effectors is the suppression of ETI in order to circumvent plant defenses (Jones and Dangl, 2006). In some cases, an effector, which suppresses the AVR activity of another effector, can itself be recognized by an R gene, thus allowing mechanistic-based strategies to genetically control plant diseases. Two such cases of AVR genes hiding another AVR gene have been reported in L. maculans and F. oxysporum.
L. maculans avirulence gene AvrLm3 is recognized by Rlm3. This recognition is suppressed in presence of AvrLm4-7 which is itself recognized by Rlm4 and Rlm7. Indeed, silencing of AvrLm4-7 in an isolate virulent toward Rlm3 allowed recognition by Rlm3, and the complementation of an isolate avirulent toward Rlm3 with AvrLm4-7 conferred virulence on Rlm3 cultivars (Plissonneau et al., 2016), confirming the ability of AvrLm4-7 to suppress AvrLm3/Rlm3-mediated resistance and the presence of AvrLm3 in L. maculans populations. AvrLm3 was recently identified and is located in a telomeric region of the L. maculans genome (Plissonneau et al., 2016). The conservation of AvrLm3 despite its telomeric location suggests an involvement of AvrLm3 in fungal fitness (Plissonneau et al., 2017). It seems that the main mechanism to acquire virulence toward Rlm3 was not the deletion of AvrLm3 but rather the production of an effector, AvrLm4-7, that conceals AvrLm3.
Fusarium oxysporum f.sp. lycopersici (Fol) is a common soil fungus infecting tomato. Several Fol AVR genes were identified, including AVR1 (recognized by R genes I and I-1), AVR2 (recognized by I-2) and AVR3 (recognized by I-3; Rep et al., 2005; Houterman et al., 2008, 2009). AVR1 is involved in the suppression of I-3 and I-2-mediated recognition of AVR3 and AVR2 respectively. Deletion of AVR1 in an isolate virulent toward I-2 and I-3 allowed recognition by I-3 and I-2 plants, and the complementation of isolates avirulent toward I-3 or I-2 with AVR1 conferred virulence on I-3 and I-2 tomato plants. AVR3 and AVR2 were shown to be essential for full virulence of Fol on tomato. In agreement, AVR3 and AVR2 are never deleted in Fol isolates, and no SNP preventing recognition by I-3 has been identified, while three SNPs preventing recognition by I-2 without altering virulence of the corresponding isolates were reported (Lievens et al., 2009). In contrast, AVR1 has no major effect on Fol virulence, suggesting that its role is mainly restricted to suppressing I-2 and I-3-mediated recognition (Houterman et al., 2008).
Such interactions offer great opportunities for the genetic control of plant diseases. In tomato, the combination of I-1and I-2/I-3 may lead to a durable resistance toward Fol, since one R gene will be effective against an AVR gene important for fungal virulence (AVR3 or AVR2) and another against the suppressor of I-3/I-2-mediated resistance. The combination of Rlm7 and Rlm3 against L. maculans could also increase the durability of the two R genes in oilseed rape. It is now important to determine whether pyramiding or alternating deployment is the best strategy. Pyramiding the two R genes will exert a strong selection pressure on fungal isolates, which could lead to the emergence of isolates virulent toward both resistances. Alternating two resistances in the field combined with a surveillance of Fol and L. maculans populations would allow counter-selection of virulent isolates.
A Bipartite Avirulence Gene Necessary for Recognition by One Resistance Gene
So far, only a single case of bipartite AVR gene/R gene interaction has been reported. In Fol, AVR2, which triggers I-2-mediated recognition and is required for full virulence on susceptible tomato (Houterman et al., 2009), shares its promoter region with SIX5, which also encodes a protein secreted in tomato xylem sap. Ma et al. (2015) recently reported that SIX5 is also required to trigger I-2-mediated recognition. Thus, deletion of SIX5 allows Fol to escape I-2-mediated resistance, while reintroduction of SIX5 restores avirulence toward I-2, showing that AVR2 and SIX5 are both necessary to induce I-2-mediated resistance. Avr2 and Six5 physically interact, suggesting that I-2 recognizes the Avr2/Six5 complex. Similar to AVR2, SIX5 is also present in all Fol isolates, and is required for full virulence on tomato (Ma et al., 2015). It is unlikely that specific resistances involved in such bipartite AVR gene/R gene interactions are more durable, since deletion or point mutation of only one of the AVR genes is sufficient to escape recognition by the corresponding R gene. Indeed, while no polymorphism was observed in the SIX5 sequence of isolates virulent toward I-2, three point mutations causing single amino acid changes were observed in AVR2, allowing Fol strains to escape I-2-mediated recognition without altering virulence.
Resistance Genes Recognizing Several Avirulence Genes in Distinct Organisms
It has been hypothesized that pathogen effectors target a common set of plant proteins and that plants have evolved surveillance systems to recognize multiple AVR genes sharing the same plant target (Mukhtar et al., 2011). Several R genes able to recognize distinct pathogens have been reported, which potentially decreases the need for chemical interventions and opens the path to broad-spectrum disease control. A notable example is Cf2 from tomato, which confers resistance to both the fungal pathogen Cladosporium fulvum and the nematode Globodera rostochiensis (Rooney et al., 2005; Lozano-Torres et al., 2012).
Several apoplastic effectors of oomycetes, fungi, bacteria and nematodes were reported to target papain-like cysteine proteases (PLCP; Kaschani et al., 2010; Lozano-Torres et al., 2012). Avr2, from the tomato leaf mold agent C. fulvum, targets the tomato PLCP Rcr3 and inhibits its activity. Its effector activity on Rcr3 is indirectly recognized by the tomato R gene Cf2, according to the guard model (Rooney et al., 2005). Cys protease activity profiling showed that Avr2 inhibited multiple extracellular Cys proteases, including Rcr3 and its close relative Pip1, and it was proposed by van der Hoorn and Kamoun (2008) that Pip1 was the operative target of Avr2 and Rcr3 acted as a decoy. Silencing of Avr2 significantly decreased C. fulvum virulence on tomato (van Esse et al., 2008). Interestingly, Rcr3 is also targeted by effectors from other pathogens. For example, an effector of the nematode G. rostochiensis, Gr-VAP1, physically interacts with Rcr3 and triggers a Cf2-dependent hypersensitive response in tomato (Lozano-Torres et al., 2012). Broad-spectrum resistances exert a strong selection pressure on pathogen populations, potentially leading to them being rapidly overcome. Indeed, even though Avr2 was demonstrated to be important for virulence, isolates of C. fulvum virulent toward Cf2 were rapidly reported (Luderer et al., 2002). However, Cf2 is still effective as a result of pyramiding with other specific R genes in tomato crops (de Wit, 2016).
Concluding Remarks
While complex interactions between bacterial AVR genes and plant R genes have been previously discovered and well-studied (Cui et al., 2009; Khan et al., 2016), the characterization of plant/fungal interactions are emerging and show some similarities (cooperating R genes, R genes recognizing distinct pathogens, AVR gene suppressing recognition of another AVR gene) but also specificities (bipartite AVR gene). Among the R genes displaying complex interaction with AVR genes, some of the most promising are those conferring broad-spectrum resistances since they guard key components of plant immunity and, as such, target essential effectors. Even if they exert a strong selection pressure on pathogen populations, they may remain effective through pyramiding with other specific or quantitative R genes. Another promising strategy to manage durable resistances would be to target antagonistic interactions between AVR genes and to combine the corresponding R genes in the same cultivars through pyramiding or to sequentially use the R genes in rotation. Although antagonistic interactions between AVR genes have only been reported twice in plant-fungi pathosystems, they are probably more widely distributed than suspected. Indeed, in cereal powdery mildews it has been suggested that pairs of AVR genes and suppressors of AVR gene recognition could form the basis of specificity (Bourras et al., 2015, 2016).
Authors Contributions
Both authors reviewed litterature, contributed to writing the manuscript, and approved it for publication.
Funding
YP was funded by a Young Scientist Funding by INRA. This work was supported by ANR (StructuraLEP project; ANR-14-CE19-0019).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge Richard O'Connell (UMR Bioger, INRA, Grignon) for critical review and polishing of the manuscript.
References
Balesdent, M.-H., Plissonneau, C., Coudard, L., Daverdin, G., Le Meur, L., Carpezat, J., et al. (2015). Résistance du colza au phoma : où en est l'efficacité de Rlm7 ? Phytoma 684, 20–24.
Blondeau, K., Blaise, F., Graille, M., Kale, S. D., Linglin, J., Ollivier, B., et al. (2015). Crystal structure of the effector AvrLm4–7 of Leptosphaeria maculans reveals insights into its translocation into plant cells and recognition by resistance proteins. Plant J. 83, 610–624. doi: 10.1111/tpj.12913
Bourras, S., McNally, K. E., Ben-David, R., Parlange, F., Roffler, S., Praz, C. R., et al. (2015). Multiple avirulence loci and allele-specific effector recognition control the Pm3 race-specific resistance of wheat to powdery mildew. Plant Cell 27, 2991–3012. doi: 10.1105/tpc.15.00171
Bourras, S., McNally, K. E., Müller, M. C., Wicker, T., and Keller, B. (2016). Avirulence genes in cereal powdery mildews: the gene-for-gene hypothesis 2.0. Front. Plant Sci. 7:241. doi: 10.3389/fpls.2016.00241
Catanzariti, A. M., Dodds, P. N., Ve, T., Kobe, B., Ellis, J. G., and Staskawicz, B. J. (2010). The AvrM effector from flax rust has a structured C-terminal domain and interacts directly with the M resistance protein. Mol. Plant Microbe Interact. 23, 49–57. doi: 10.1094/MPMI-23-1-0049
Catanzariti, A. M., Lim, G. T., and Jones, D. A. (2015). The tomato I-3 gene: a novel gene for resistance to Fusarium wilt disease. New Phytol. 207, 106–118. doi: 10.1111/nph.13348
Cesari, S., Bernoux, M., Moncuquet, P., Kroj, T., and Dodds, P. (2014a). A novel conserved mechanism for plant NLR protein pairs: the “integrated decoy” hypothesis. Front. Plant Sci. 5:606. doi: 10.3389/fpls.2014.00606
Cesari, S., Kanzaki, H., Fujiwara, T., Bernoux, M., Chalvon, V., Kawano, Y., et al. (2014b). The NB-LRR proteins RGA4 and RGA5 interact functionally and physically to confer disease resistance. EMBO J. 33, 1941–1959. doi: 10.15252/embj.201487923
Cesari, S., Thilliez, G., Ribot, C., Chalvon, V., Michel, C., Jauneau, A., et al. (2013). The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell 25, 1463–1481. doi: 10.1105/tpc.112.107201
Cui, H., Xiang, T., and Zhou, J. M. (2009). Plant immunity: a lesson from pathogenic bacterial effector proteins. Cell. Microbiol. 11, 1453–1461. doi: 10.1111/j.1462-5822.2009.01359.x
Dangl, J. L., and Jones, J. D. (2001). Plant pathogens and integrated defence responses to infection. Nature 411, 826–833. doi: 10.1038/35081161
Daverdin, G., Rouxel, T., Gout, L., Aubertot, J. N., Fudal, I., Meyer, M., et al. (2012). Genome structure and reproductive behaviour influence the evolutionary potential of a fungal phytopathogen. PLoS Pathog. 8:e1003020. doi: 10.1371/journal.ppat.1003020
de Guillen, K., Ortiz-Vallejo, D., Gracy, J., Fournier, E., Kroj, T., and Padilla, A. (2015). Structure analysis uncovers a highly diverse but structurally conserved effector family in phytopathogenic fungi. PLoS Pathog. 11:e1005228. doi: 10.1371/journal.ppat.1005228
Delourme, R., Pilet-Nayel, M. L., Archipiano, M., Horvais, R., Tanguy, X., Rouxel, T., et al. (2004). A cluster of major specific resistance genes to Leptosphaeria maculans in Brassica napus. Phytopathology 94, 578–583. doi: 10.1094/PHYTO.2004.94.6.578
de Wit, P. J. G. M. (2016). Cladosporium fulvum effectors: weapons in the arms race with tomato. Annu. Rev. Phytopathol. 4, 1–23. doi: 10.1146/annurev-phyto-011516-040249
Dodds, P. N., Lawrence, G. J., Catanzariti, A. M., The, T., Wang, C. I., Ayliffe, M. A., et al. (2006). Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl. Acad. Sci. U.S.A. 103, 8888–8893. doi: 10.1073/pnas.0602577103
Farman, M. L., Eto, Y., Nakao, T., Tosa, Y., Nakayashiki, H., Mayama, S., et al. (2002). Analysis of the structure of the AVR1-CO39 avirulence locus in virulent rice-infecting isolates of Magnaporthe grisea. Mol. Plant Microbe Interact. 15, 6–16. doi: 10.1094/MPMI.2002.15.1.6
Fisher, M. C., Henk, D. A., Briggs, C. J., Brownstein, J. S., Madoff, L. C., McCraw, S. L., et al. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. doi: 10.1038/nature10947
Gout, L., Fudal, I., Kuhn, M. L., Blaise, F., Eckert, M., Cattolico, L., et al. (2006). Lost in the middle of nowhere: the AvrLm1 avirulence gene of the Dothideomycete Leptosphaeria maculans. Mol. Microbiol. 60, 67–80. doi: 10.1111/j.1365-2958.2006.05076.x
Gout, L., Kuhn, M. L., Vincenot, L., Bernard-Samain, S., Cattolico, L., Barbetti, M., et al. (2007). Genome structure impacts molecular evolution at the AvrLm1 avirulence locus of the plant pathogen Leptosphaeria maculans. Environ. Microbiol. 9, 2978–2992. doi: 10.1111/j.1462-2920.2007.01408.x
Guttman, D. S., McHardy, A. C., and Schulze-Lefert, P. (2014). Microbial genome-enabled insights into plant-microorganism interactions. Nat. Rev. Genet. 15, 797–813. doi: 10.1038/nrg3748
Houterman, P. M., Cornelissen, B. J., and Rep, M. (2008). Suppression of plant resistance gene-based immunity by a fungal effector. PLoS Pathog. 4:e1000061. doi: 10.1371/journal.ppat.1000061
Houterman, P. M., Ma, L., van Ooijen, G., de Vroomen, M. J., Cornelissen, B. J., Takken, F. L. W., et al. (2009). The effector protein Avr2 of the xylem-colonizing fungus Fusarium oxysporum activates the tomato resistance protein I-2 intracellularly. Plant J. 58, 970–978. doi: 10.1111/j.1365-313X.2009.03838.x
Huang, Y.-J., Balesdent, M.-H., Li, Z.-Q., Evans, N., Rouxel, T., and Fitt, B. D. L. (2010). Fitness cost of virulence differs between the AvrLm1 and AvrLm4 loci in Leptosphaeria maculans (Phoma stem canker of oilseed rape). Eur. J. Plant Pathol. 126, 279–291. doi: 10.1007/s10658-009-9539-7
Huang, Y. J., Li, Z. Q., Evans, N., Rouxel, T., Fitt, B. D. L., and Balesdent, M.-H. (2006). Fitness cost associated with loss of the AvrLm4 avirulence function in Leptosphaeria maculans (phoma stem canker of oilseed rape). Eur. J. Plant Pathol. 114, 77–89. doi: 10.1007/s10658-005-2643-4
Jia, Y., McAdams, S. A., Bryan, G. T., Hershey, H. P., and Valent, B. (2000). Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 19, 4004–4014. doi: 10.1093/emboj/19.15.4004
Jones, J. D., and Dangl, J. L. (2006). The plant immune system. Nature 444, 323–329. doi: 10.1038/nature05286
Kanzaki, H., Yoshida, K., Saitoh, H., Fujisaki, K., Hirabuchi, A., Alaux, L., et al. (2012). Arms race co-evolution of Magnaporthe oryzae AVR-Pik and rice Pik genes driven by their physical interactions. Plant J. 72, 894–907. doi: 10.1111/j.1365-313X.2012.05110.x
Kaschani, F., Shabab, M., Bozkurt, T., Shindo, T., Schornack, S., Gu, C., et al. (2010). An effector-targeted protease contributes to defense against Phytophthora infestans and is under diversifying selection in natural hosts. Plant Physiol. 154, 1794–1804. doi: 10.1104/pp.110.158030
Keen, N. T. (1990). Gene-for-gene complementarity in plant-pathogen interactions. Annu. Rev. Genet. 24, 447–463. doi: 10.1146/annurev.ge.24.120190.002311
Khan, M., Subramaniam, R., and Desveaux, D. (2016). Of guards, decoys, baits and traps: pathogen perception in plants by type III effector sensors. Curr. Opin. Microbiol. 29, 49–55. doi: 10.1016/j.mib.2015.10.006
Kim, S. H., Qi, D., Ashfield, T., Helm, M., and Innes, R. W. (2016). Using decoy to expand the recognition specificity of a plant disease resistance protein. Science 351, 684–687. doi: 10.1126/science.aad3436
Kroj, T., Chanclud, E., Michel-Romiti, C., Grand, X., and Morel, J. B. (2016). Integration of decoy domains derived from protein targets of pathogen effectors into plant immune receptors is widespread. New Phytol. 210, 318–626. doi: 10.1111/nph.13869
Larkan, N. J., Lydiate, D. J., Parkin, I. A., Nelson, M. N., Epp, D. J., Cowling, W. A., et al. (2013). The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AvrLm1. New Phytol. 197, 595–605. doi: 10.1111/nph.12043
Le Roux, C., Jauneau, A., Camborde, L., Trémousaygue, D., Kraut, A., Zhou, B., et al. (2015). A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell 161, 1074–1088. doi: 10.1016/j.cell.2015.04.025
Lievens, B., Houterman, P. M., and Rep, M. (2009). Effector gene screening allows unambiguous identification of Fusarium oxysporum f. sp. lycopersici races and discrimination from other formae speciales. FEMS Microbiol. Lett. 300, 201–215. doi: 10.1111/j.1574-6968.2009.01783.x
Liu, W., Liu, J., Ning, Y., Ding, B., Wang, X., Wang, Z., et al. (2013). Recent progress in understanding PAMP- and effector-triggered immunity against the rice blast fungus Magnaporthe oryzae. Mol. Plant 6, 605–620. doi: 10.1093/mp/sst015
Lozano-Torres, J. L., Wilbers, R. H. P., Gawronski, P., Boshoven, J. C., Finkers-Tomczak, A., Cordewener, J. H., et al. (2012). Dual disease resistance mediated by the immune receptor Cf-2 in tomato requires a common virulence target of a fungus and a nematode. Proc. Natl. Acad. Sci. U.S.A. 109, 10119–10124. doi: 10.1073/pnas.1202867109
Luderer, R., Takken, F. L., de Wit, P. J., and Joosten, M. H. (2002). Cladosporium fulvum overcomes Cf-2-mediated resistance by producing truncated AVR2 elicitor proteins. Mol. Microbiol. 45, 875–884. doi: 10.1046/j.1365-2958.2002.03060.x
Ma, L., Houterman, P. M., Gawehns, F., Cao, L., Sillo, F., Richter, H., et al. (2015). The AVR2–SIX5 gene pair is required to activate I-2-mediated immunity in tomato. New Phytol. 208, 507–518. doi: 10.1111/nph.13455
Maqbool, A., Saitoh, H., Franceschetti, M., Stevenson, C. E., Uemura, A., Kanzaki, H., et al. (2015). Structural basis of pathogen recognition by an integrated HMA domain in a plant NLR immune receptor. Elife 4:e08709. doi: 10.7554/eLife.08709
Mukhtar, M. S., Carvunis, A.-R., Dreze, M., Epple, P., Steinbrenner, J., Moore, J., et al. (2011). Independently evolved virulence effectors converge onto hubs in a plant immune system network. Science 333, 596–601. doi: 10.1126/science.1203659
Okuyama, Y., Kanzaki, H., Abe, A., Yoshida, K., Tamiru, M., Saitoh, H., et al. (2011). A multifaceted genomics approach allows the isolation of the rice Pia-blast resistance gene consisting of two adjacent NBS-LRR protein genes. Plant J. 66, 467–479. doi: 10.1111/j.1365-313X.2011.04502.x
Oliva, R., Win, J., Raffaele, S., Boutemy, L., Bozkurt, T. O., et al. (2010). Recent developments in effector biology of filamentous plant pathogens. Cell. Microbiol. 12, 705–715. doi: 10.1111/j.1462-5822.2010.01471.x
Ortiz, D., Guillen, K. D., Cesari, S., Chalvon, V., Gracy, J., Padilla, A., et al. (2017). Recognition of the Magnaporthe oryzae effector AVR-Pia by the decoy domain of the rice NLR immune receptor RGA5. Plant Cell 29, 156–168. doi: 10.1105/tpc.16.00435
Parlange, F., Daverdin, G., Fudal, I., Kuhn, M. L., Balesdent, M. H., Blaise, F., et al. (2009). Leptosphaeria maculans avirulence gene AvrLm4-7 confers a dual recognition specificity by the Rlm4 and Rlm7 resistance genes of oilseed rape, and circumvents Rlm4-mediated recognition through a single amino acid change. Mol. Microbiol. 71, 851–863. doi: 10.1111/j.1365-2958.2008.06547.x
Plissonneau, C., Blaise, F., Ollivier, B., Leflon, M., Carpezat, J., Rouxel, T., et al. (2017). Unusual evolutionary mechanisms to escape effector-triggered-immunity in the fungal phytopathogen Leptosphaeria maculans. Mol. Ecol. 26, 2183–2198. doi: 10.1111/mec.14046
Plissonneau, C., Daverdin, G., Ollivier, B., Blaise, F., Degrave, A., Fudal, I., et al. (2016). A game of hide and seek between avirulence genes AvrLm4-7 and AvrLm3 in Leptosphaeria maculans. New Phytol. 209, 1613–1624. doi: 10.1111/nph.13736
Rep, M., Meijer, M., Houterman, P. M., van der Does, H. C., and Cornelissen, B. J. (2005). Fusarium oxysporum evades I-3-mediated resistance without altering the matching avirulence gene. Mol. Plant Microbe Interact. 18, 15–23. doi: 10.1094/MPMI-18-0015
Ribot, C., Césari, S., Abidi, I., Chalvon, V., Bournaud, C., Vallet, J., et al. (2013). The Magnaporthe oryzae effector AVR1–CO39 is translocated into rice cells independently of a fungal-derived machinery. Plant J. 74, 1–12. doi: 10.1111/tpj.12099
Rooney, H. C., van't Klooster, J. W., van der Hoorn, R. A., Joosten, M. H., Jones, J. D., and de Wit, P. J. (2005). Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf-2-dependent disease resistance. Science 308, 1783–1786. doi: 10.1126/science.1111404
Rouxel, T., and Balesdent, M. H. (2017). Life, death and rebirth of avirulence effectors in a fungal pathogen of Brassica crops, Leptosphaeria maculans. New Phytol. 214, 526–532. doi: 10.1111/nph.14411
Rouxel, T., Penaud, A., Pinochet, X., Brun, H., Gout, L., Delourme, R., et al. (2003). A 10-year survey of populations of Leptosphaeria maculans in France indicates a rapid adaptation towards the Rlm1 resistance gene of oilseed rape. Eur. J. Plant Pathol. 109, 871–881. doi: 10.1023/A:1026189225466
Sarris, P. F., Duxbury, Z., Ma, Y., Segonzac, C., Sklenar, J., Derbyshire, P., et al. (2015). A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell 161, 1089–1100. doi: 10.1016/j.cell.2015.04.024
Sprague, S. J., Marcroft, S. J., Hayden, H. L., and Howlett, B. J. (2006). Major gene resistance to blackleg in Brassica napus overcome within three years of commercial production in Southeastern Australia. Plant Dis. 90, 190–198. doi: 10.1094/PD-90-0190
Steinbrenner, A. D., Goritschnig, S., and Staskawicz, B. J. (2015). Recognition and activation domains contribute to allele-specific responses of an Arabidopsis NLR receptor to an oomycete effector protein. PLoS Pathog. 11:e1004665. doi: 10.1371/journal.ppat.1004665
Takagi, H., Uemura, A., Yaegashi, H., Tamiru, M., Abe, A., Mitsuoka, C., et al. (2013). MutMap-Gap: whole-genome resequencing of mutant F2 progeny bulk combined with de novo assembly of gap regions identifies the rice blast resistance gene Pii. New Phytol. 200, 276–283. doi: 10.1111/nph.12369
Takken, F. L., and Goverse, A. (2012). How to build a pathogen detector: structural basis of NB-LRR function. Curr. Opin. Plant Biol. 15, 375–384. doi: 10.1016/j.pbi.2012.05.001
van der Hoorn, R. A., and Kamoun, S. (2008). From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell 20, 2009–2017. doi: 10.1105/tpc.108.060194
van Esse, H. P., van't Klooster, J. W., Bolton, M. D., Yadeta, K. A., van Baarlen, P., Boeren, S., et al. (2008). The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defense. Plant Cell 20, 1948–1963. doi: 10.1105/tpc.108.059394
Yang, X., Deng, F., and Ramonell, K. M. (2012). Receptor-like kinases and receptor-like proteins: keys to pathogen recognition and defense signaling in plant innate immunity. Front. Biol. 7, 155–166. doi: 10.1007/s11515-011-1185-8
Yoshida, K., Saitoh, H., Fujisawa, S., Kanzaki, H., Matsumura, H., Yoshida, K., et al. (2009). Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell 21, 1573–1591. doi: 10.1105/tpc.109.066324
Keywords: avirulence genes, resistance genes, fungal effectors, resistance management, virulence factors
Citation: Petit-Houdenot Y and Fudal I (2017) Complex Interactions between Fungal Avirulence Genes and Their Corresponding Plant Resistance Genes and Consequences for Disease Resistance Management. Front. Plant Sci. 8:1072. doi: 10.3389/fpls.2017.01072
Received: 03 January 2017; Accepted: 02 June 2017;
Published: 16 June 2017.
Edited by:
Fabienne Vailleau, Institut National De La Recherche Agronomique Centre Occitanie-Toulouse, FranceReviewed by:
Maud Bernoux, Commonwealth Scientific and Industrial Research Organisation (CSIRO), AustraliaIsabel M. L. Saur, Max Planck Institute for Plant Breeding Research (MPG), Germany
Copyright © 2017 Petit-Houdenot and Fudal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabelle Fudal, isabelle.fudal@inra.fr
 Yohann Petit-Houdenot
Yohann Petit-Houdenot Isabelle Fudal
Isabelle Fudal