Olfactory and cortical projections to bulbar and hippocampal adult-born neurons
- 1Neuroplasticity and Neurodegeneration Laboratory, Department of Medical Sciences, Ciudad Real Medical School, El Centro Regional de Investigaciones Biomédicas, Universidad de Castilla-La Mancha, Ciudad Real, Spain
- 2Department of Cellular and Molecular Neurobiology, Neurobiology Institute, Universidad Nacional Autónoma de México, Querétaro, México
- 3Departamento de Biología Celular, Facultad de Ciencias Biológicas, Universidad de Valencia, Burjassot, Spain
New neurons are continually generated in the subependymal layer of the lateral ventricles and the subgranular zone of dentate gyrus during adulthood. In the subventricular zone, neuroblasts migrate a long distance to the olfactory bulb where they differentiate into granule or periglomerular interneurons. In the hippocampus, neuroblasts migrate a short distance from the subgranular zone to the granule cell layer of the dentate gyrus to become granule neurons. In addition to the short-distance inputs, bulbar interneurons receive long-distance centrifugal afferents from olfactory-recipient structures. Similarly, dentate granule cells receive differential inputs from the medial and lateral entorhinal cortices through the perforant pathway. Little is known concerning these new inputs on the adult-born cells. In this work, we have characterized afferent inputs to 21-day old newly-born neurons. Mice were intraperitoneally injected with bromodeoxyuridine. Two weeks later, rhodamine-labeled dextran-amine was injected into the anterior olfactory nucleus, olfactory tubercle, piriform cortex and lateral and medial entorhinal cortices. One week later, animals were perfused and immunofluorescences were carried out. The data show that projection neurons from the mentioned structures, establish putative synaptic contacts onto 21-day-old neurons in the olfactory bulb and dentate gyrus, in some cases even before they start to express specific subpopulation proteins. Long-distance afferents reach middle and outer one-third portions of the molecular layer of the dentate gyrus and granule and, interestingly, periglomerular layers of the olfactory bulb. In the olfactory bulb, these fibers appear to establish presumptive axo-somatic contacts onto newly-born granule and periglomerular cells.
Introduction
The subventricular zone of the lateral ventricles (SVZ) and the subgranular zone (SGZ) of the dentate gyrus (DG) were described as the two main neurogenic niches of the adult mammalian forebrain (Altman, 1962; Altman and Das, 1965; Luskin, 1993; Lois and Alvarez-Buylla, 1994). Decades later, the functional significance of new neurons integrated into adult olfactory bulb (OB) and DG is only partially known (Aimone et al., 2010; Sahay et al., 2011; Sakamoto et al., 2014).
In the SVZ, neuroblasts migrate tangentially during about 7 days through the rostral migratory stream to the OB (Alvarez-Buylla and Garcia-Verdugo, 2002) following 2 major signals, structural (astrocytes, vasculature and extracellular matrix) and molecular (BDNF, Netrins, Slits, etc.) factors (Nguyen-Ba-Charvet et al., 2004; Whitman et al., 2009; Kaneko et al., 2010). There, they migrate radially to mature into granule (Petreanu and Alvarez-Buylla, 2002) or periglomerular (Carlen et al., 2002; Lledo et al., 2006) interneurons 15 (Petreanu and Alvarez-Buylla, 2002) and 28 (Belluzzi et al., 2003) days after birth, respectively. In the DG, neuroblasts migrate a short distance from the SGZ to the granule cell layer to become fully functional neurons after 4–28 days (Van Praag et al., 2002).
This differentiation and integration implies hodological and functional changes (Petreanu and Alvarez-Buylla, 2002; Lledo and Saghatelyan, 2005; Lepousez et al., 2013). Projections from bulbar mitral and tufted cells to olfactory-recipient areas in the basal telencephalon (Pro-Sistiaga et al., 2007; Martinez-Marcos, 2009) are reciprocated by centrifugal projections to the bulb arising from the anterior olfactory nucleus (AON), the olfactory tubercle (OT), the olfactory amygdala and the piriform (PIR) and lateral entorhinal (LEC) cortices (Mohedano-Moriano et al., 2012). Newly-born olfactory granule cells first receive intrinsic GABAergic synaptic connections (3–7 days after birth) and extrinsic glutamatergic inputs thereafter (Belluzzi et al., 2003; Carleton et al., 2003; Panzanelli et al., 2009; Lepousez et al., 2013). New neurons integrate into functional circuits in a “listen before talk” manner (Whitman and Greer, 2009). These centrifugal inputs and the ambient GABA signals may play an important role in maintenance and synaptic integration by regulation of dendritic growth of newly generated neurons in preexisting adult neuronal networks (Gascon et al., 2006; Mouret et al., 2009; Pallotto and Deprez, 2014). Interestingly, LEC send centrifugal inputs to the OB and also projects to the hippocampus.
Dentate granule cells receive, through the perforant path, massive afferents from the lateral (LEC) and medial (MEC) entorhinal cortices (Steward, 1976; Witter, 2007), which ends in the outer and middle one-thirds of the molecular layer of the DG, respectively (Ramón Y Cajal, 1893; Hjorth-Simonsen, 1972; Hjorth-Simonsen and Jeune, 1972). Newly-born dentate granule cells initially receive excitatory GABAergic inputs from local interneurons, and this becomes inhibitory only once the glutamatergic extrinsic input has established, 2 weeks after birth (Overstreet Wadiche et al., 2005; Toni et al., 2007). In the same manner, these inputs seem to play an essential role for cell survival, maturation and maintenance of new dentate granule cells in the hippocampal circuitry (Van Der Borght et al., 2005; Tashiro et al., 2006; Li et al., 2012).
Therefore, in the present work we have tried to characterize inputs arising from different structures onto 21 day-old adult-born granule cells in the OB and DG of adult mice. These data could help to understand the anatomical and physiological changes underlying the integration of neurogenic elements in the adult brain.
Material and Methods
Experimental Animals
Thirty adult (6 weeks) mice (C57BL/J) of both sexes (15/15) were obtained from Charles River (Barcelona, Spain) and maintained under controlled temperature and a 12:12 h light/dark cycle with food and water ad libitum. Experimental procedures were carried out according to guidelines of the Spanish (RD53/2013) and European (Directive 2010/63/EU) legislation of the protection of animals used for experimental purpose, and the Ethical Committee of Animal Research of the University of Castilla-La Mancha.
Bromodeoxyuridine Administration
BrdU (5-bromo-2′-deoxyuridine, Fluka, Madrid, Spain) administration included 4 ip doses (at 2-h intervals) of 10 mg/mL BrdU in phosphate-buffered saline (PBS, 0.15 M NaCl, 0.01 M sodium phosphate pH 7.4) totalizing a dose of 200 mg/kg in 1 day. This dose was employed following previous results in our laboratory to optimize labeling without increasing apoptosis (Martinez-Marcos et al., 2005; De La Rosa-Prieto et al., 2009a).
Tracer Injections and Perfusion
Two weeks after BrdU administration, animals were anesthetized with a combined dose of ketamine hydrochloride (Ketolar, Parke-Davis, Madrid, Spain, 1.5 mL/kg, 75 mg/kg) and xylazine (Xilagesic, Calier, Barcelona, Spain, 0.5 mL/kg, 10 mg/kg). Under stereotaxic control, rhodamine-labeled dextran-amine (RDA, 10,000 mW, lysine fixable, Molecular Probes, Eugene, OR; 10% diluted in PBS) was ionophoretically injected (30–80 μm diameter tip; positive current pulses 7/7 s; 2–7 μA; 8–20 min) at the intended injection sites. Five experimental groups (n = 6, 3 M, F) totalizing N = 30 rodents were established according to coordinates relative to Bregma: AON (anterior 2.6; lateral 1; depth 2.7), OT (anterior 1.1; lateral 1.5; depth 4.7), PC (anterior 1.1; lateral 2.5; depth 4), LEC (posterior −4.36; lateral 4; depth 2), and MEC (posterior −4.5; lateral 3.3; depth 3) (Franklin and Paxinos, 2008). In all experimental groups (n = 6), a minimum of five cases included injections restricted at intended site and, therefore, comparable.
One week afterwards, animals were anesthetized (as above) and perfused with saline solution followed by 4% w/v paraformaldehyde fixative in phosphate buffer (0.1 M sodium phosphate pH 7.2). Brains were postfixed in 4% w/v paraformaldehyde, cryoprotected in 30% w/v sucrose, and sagittally (olfactory bulb) or frontally sectioned (50 μm) using a freezing sliding microtome (Microm HM450). Sections were consecutively collected into 96-well plates and maintained at 4°C in preserving solution (PBS containing 20% v/v glycerol and 30% v/v ethylene glycol) for further processing.
Immunofluorescence Procedures
Sections were rinsed overnight with Tris-buffered saline (TBS; 0.15 M NaCl, 0.05 M Tris, HCl pH 7.6), fixed with 4% w/v paraformaldehyde in phosphate buffer (PB; 0.1 M sodium phosphate pH 7.2) for 30 min at 37°C, rinsed in TBS (3 × 5 min), treated with 37% w/v HCl for 20 min at 37°C and then with pepsin 0.5 mg/mL in 37% w/v HCl for 20 min at 37°C. Sections were rinsed (3 × 10 min) and blocked with 10% normal donkey serum (Vector Laboratories, Burlingame, CA) in TBS for 30 min. Sections were then incubated overnight with mouse anti-BrdU (1:40, Dako, M0744, Glostrup, Denmark) or rat anti-BrdU (1:40; Santa Cruz, SC-70441, CA, USA) and rabbit anti-calretinin (CR, 1:500, Swant, Bellinzona, Switzerland), goat anti-doublecortin (DCX, 1:200, Santa Cruz, sc-8066, CA, USA) and rabbit anti-postsynaptic density-95 (PSD-95, 1:50, Santa Cruz, sc-28941, CA, USA) antibodies diluted in TBS with 1% v/v normal goat serum at 4°C. Antibodies specificity was assessed by using untreated animals and/or omitting primary antibodies. Sections were rinsed and incubated with Alexa 405 anti-mouse, Alexa 488 anti-mouse or anti-rat, Alexa 594 anti-rabbit and Alexa 647 anti-goat (1:200 in TBS with 2% of normal goat serum and 0.2% Triton X-100; Invitrogen, Eugene, OR), and counterstained using DAPI (1 μg/ml in TBS, Santa Cruz, SC-3598) for 5 min in the dark. All sections were mounted and coverslipped with PVA-DABCO solution (DABCO®).
Analysis of Labeled Cells
Sections under study were analyzed using epifluorescence (Nikon eclipse Ti) and confocal microscopy (Zeiss LSM 710). Brightness, contrast and gamma of images were adjusted using Irfanview software and figures arranged and lettered using Canvas software.
Results
Injection Sites
In order to label afferent connections to the olfactory bulb arising from olfactory-recipient structures (Mohedano-Moriano et al., 2012) and to the dentate gyrus originating in the entorhinal cortex, injections were aimed at the AON (Figure 1A), OT (Figure 1B), PIR (Figure 1B), LEC (Figure 1C) and MEC (Figure 1D), respectively. The resulting anterograde labeling was analyzed in the different layers of the olfactory bulb (Figure 1E), particularly in the granule cell (GrL) and glomerular (GL) layers (Figure 1F) as well as in the caudoventral DG (Figure 1G), specifically in the molecular layer (Figure 1H).
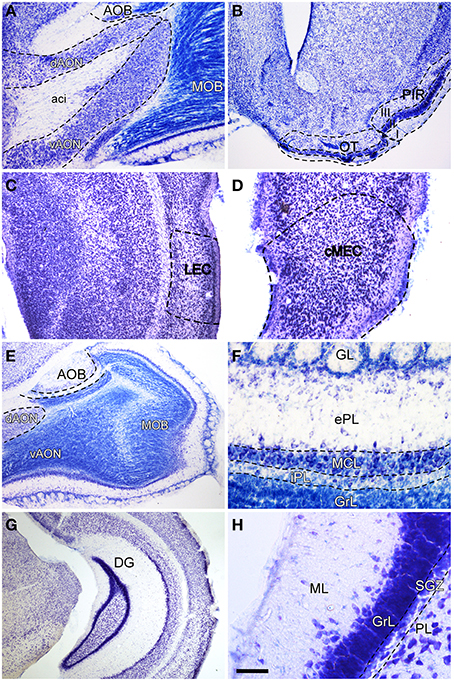
Figure 1. Nissl-stained coronal (B–D,G,H) and sagittal (A,E,F) sections of olfactory bulb and dentate gyrus. Aci, anterior commissure intrabulbar; AOB, accessory olfactory bulb; cMEC, caudo-medial entorhinal cortex; dAON, anterior olfactory nucleus dorsal; DG, dentate gyrus; ePL, external plexiform layer olfactory bulb; GL, glomerular layer olfactory bulb; GrL, granular layer; iPL, internal plexiform layer olfactory bulb; LEC, lateral entorhinal cortex; MCL, mitral cell layer; ML, molecular layer dentate gyrus; MOB, main olfactory bulbs; OT, olfactory tubercle; PIR, piriform cortex; PL, polymorph layer of the dentate gyrus; SGZ, subgranular zone; vAON, anterior olfactory nucleus ventral. Calibration bar for (A) 334 μm; (B y G) 500 μm; (C y E) 250 μm; (D) 167 μm; (F) 100 μm; (H) 50 μm.
Injections of RDA in the AON involved both dorsal and ventral components of the nucleus as well as the intrabulbar portion of the anterior commissure (Figures 2A,B). Injections in the OT mostly involved cell layer III including islands of Calleja (Figures 2C,D). Tracer deposits in the PIR were centered in cell layers II and III (Figures 2E,F). Similarly, injections in LEC mostly involved supragranular cell layers II and III (Figures 2G,H); whereas injections in MEC were centered in infragranular cell layers IV-VI (Figures 2I,J).
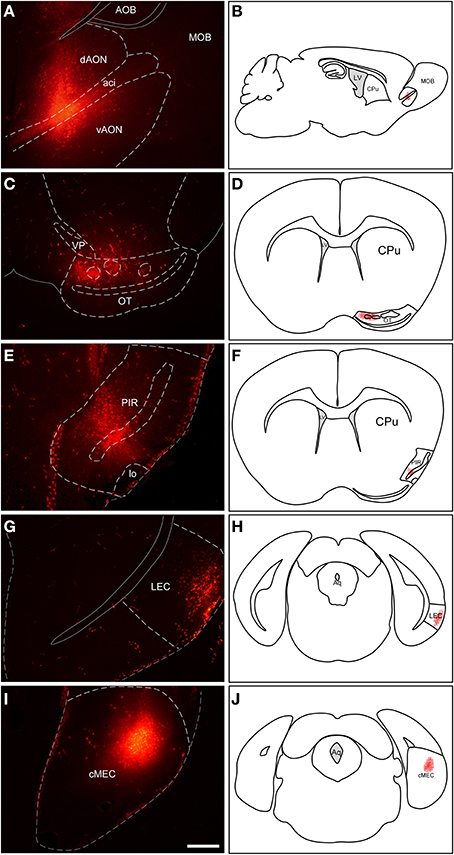
Figure 2. Injection sites of rhodamine-labeled dextran-amine (RDA) tracer in anterior olfactory nucleus (A,B), olfactory tubercle (C,D), piriform cortex (E,F), lateral entorhinal cortex (G,H) and medial entorhinal cortex (I,J). Aci, anterior commissure intrabulbar; AOB, accessory olfactory bulb; cMEC, caudo-medial entorhinal cortex; CPu, caudatus putamen; dAON, anterior olfactory nucleus dorsal; LEC, lateral entorhinal cortex; lo, lateral olfactory tract; LV, lateral ventricle; MOB, main olfactory bulbs; OT, olfactory tubercle; PIR, piriform cortex; vAON, anterior olfactory nucleus ventral; VP, ventral pallidum. Calibration bar for (A,C,E,G,I) 500 μm.
Anterograde Labeling
Tracer injections in the AON (Figures 2A,B) resulted in anterogradely labeled fibers in the olfactory bulb. Confocal microscopy showed abundant, thin fibers concentrated in the granule cell layer (Figure 3A) as evidenced by double labeling with calretinin (CR) (Figure 3B). High-power images revealed labeled fibers around glomeruli in the glomerular layer (Figure 3C).
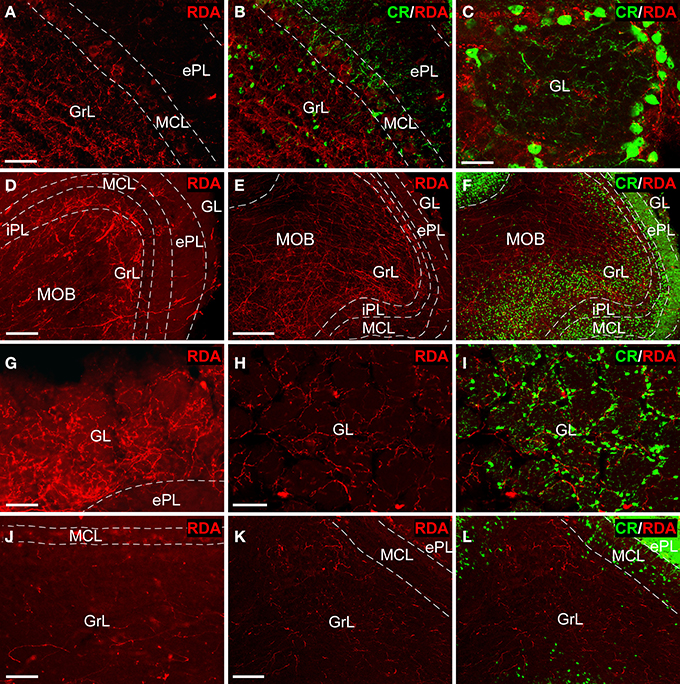
Figure 3. Tract-tracing and immunofluorescence experiments. Sagittal sections of the anterior olfactory bulb showing incoming rhodamine-labeled dextran-amine (RDA)-fibers from injections into the anterior olfactory nucleus (A–C), olfactory tubercle (D–I) and piriform cortex (J–L) showing a rhodamine-labeled dextran-amine (RDA) under epifluorescence (D,G,J) and confocal (A,B,C,E,F,H,I,K,L) microscopy. CR, calretinin; ePL, external plexiform layer olfactory bulb; GL, glomerular layer olfactory bulb; GrL, granular layer; iPL, internal plexiform layer olfactory bulb; MCL, mitral cell layer; MOB, main olfactory bulbs. Calibration bar for (A,B) 25 μm; C 10 μm; (D) 100 μm; (E,F) 200 μm; (G–L) 50 μm.
Injections in the OT (Figures 2C,D) yielded thick, labeled fibers under fluorescence (Figure 3D) and confocal (Figure 3E) microscopy throughout all layers of the OB as evidenced by CR expression (Figure 3F). Terminal-like fibers concentrated in the GL (Figure 3G) where they exhibited varicosities (Figure 3H). Double labeling experiments with CR demonstrated that most of these fibers were arranged in a periglomerular pattern (Figures 3C,I).
Tracer deposits in the PIR (Figures 2E,F) resulted in scarce, thin fibers mostly distributed into the GrL (Figure 3J). Confocal microscopy revealed that these fibers occasionally showed varicosities (Figure 3K). The preferential expression in the granule cell layer was assessed by CR immunoreactivity (Figure 3L).
Experiments with RDA in the enthorinal cortex, aimed at the LEC (Figures 2G,H) and MEC (Figures 2I,J) labeled fibers along the perforant path that formed plexuses in the DG. Specifically, injections in LEC gave rise a band of labeled, varicose fibers in outer one-third of the molecular layer of the DG (Figure 4A); whereas injections in the MEC yielded a band of varicose fibers in the middle one-third of the molecular layer (Figure 4B). This labeling was only observed in all cases in the most caudoventral DG, from −3 to −4 mm posterior from bregma (Franklin and Paxinos, 2008).
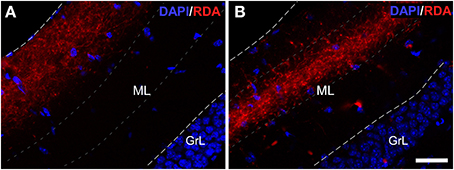
Figure 4. Double immunofluorescence for DAPI (blue) and rhodamine-labeled dextran-amine (RDA) (red) showing lateral (LEC) and medial (MEC) entorhinal cortices. LEC (A) and MEC (B) end in the outer and middle one-thirds of the molecular layer (ML) of the dentate gyrus, respectively. Calibration bar for (A,B) 25 μm. GrL: granular layer.
Immunofluorescence
Confocal z-stacks images after injections in the AON (Figures 2A,B), showed BrdU-labeled nuclei in the GL surrounded by terminal fibers indicating presumptive axo-somatic synaptic contacts (Figures 5A–D). In the GrL, BrdU-labeled cells (Figure 5E), co-expressing CR (Figure 5F), were in close proximity to RDA-labeled fibers (Figure 5G), suggesting also putative axo-somatic contacts onto double labeled cells (Figure 5H). In experiments including RDA injections in the OT (Figures 2C,D), BrdU-labeled cells (Figure 5I) also expressed CR (Figure 5J) and RDA-labeled fibers (Figure 5K) suggesting contacts on the cell body of these cells (Figure 5L). In the cases with deposits in the PIR (Figures 2E,F), some BrdU-labeled cells (Figure 5M) co-expressed CR (asterisks in Figure 5N) and some others do not (arrowheads in Figure 5N). Fibers labeled with RDA (Figure 5O) appears to reach BrdU-labeled cells (arrowheads in Figure 5P) as well as BrdU- and CR-expressing cells (asterisk in Figure 5P). High magnification images demonstrated that 21 day-old BrdU-labeled cells (Figure 5Q), even not co-expressing CR (Figure 5R), were surrounded by labeled, beaded, terminal fibers (Figure 5S), establishing putative contacts (Figure 5T).
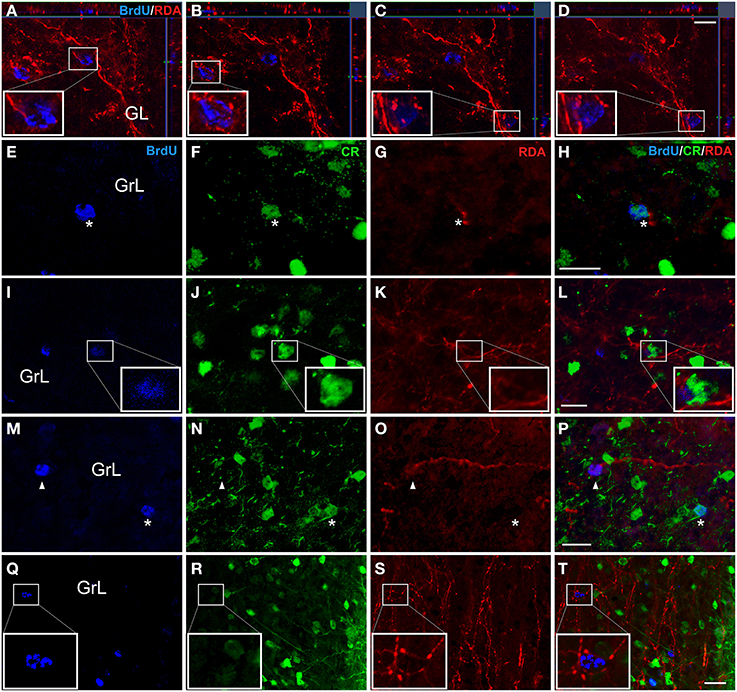
Figure 5. Double and triple immunofluorescence againts bromodeoxyuridine (BrdU) (blue), calretinin (CR) (green) and rhodamine-labeled dextran-amine (RDA) (red). Asterisks indicate newly-born olfactory granule cells co-expressing BrdU and CR and establishing contacts with RDA-labeled terminal fibers from AON (A-H), OT (I-L) and PIR (M-T). Arrowheads show contacts between BrdU+ cells and RDA-labeled fibers. Calibration bar for (A–D) 10 μm, (E–T) 20 μm.
Triple labeling experiments in the DG including injections in the entorhinal cortex combined with synaptic (Kosel et al., 1981) and maturational markers reveal hodological changes in newly-born neurons. Experiments including RDA injections in MEC (Figures 2I,J) reveal BrdU-positive cells in the SGZ (Figure 6A) and RDA-labeled fibers in the middle one-third of the molecular layer (Figure 6B). Interestingly, the expression of the postsynaptic marker PSD-95 (Figure 6C) matches that of RDA-labeled fibers (Figure 6D) thus suggesting presumptive synaptic contacts. The expression of doublecortin (DCX), a marker of neuroblasts during maturation and even maturation –up to 1 month after birth (Torubarova et al., 1981)-, shows the dendritic tree of young neurons occupying the full extent of the molecular layer (Figures 6E,F). Expression of PSD-95 (Figure 6G) (Kosel et al., 1981) suggests synaptic contacts on these cells (Figure 6H). Occasionally, BrdU-labeled cells (arrow in Figure 6I) and the resulting RDA labeling (Figure 6J) combined with DCX expression (arrowheads in Figure 6K) revealed BrdU-labeled cells (arrow in Figure 6L) displaying an apical dendrite (arrowheads in Figure 6L) reaching the zone of RDA-labeled fibers after injections in the MEC (Figure 6L). Because of BrdU protocol using HCl, immunodetection of some proteins, including PSD-95 and DCX becomes less sensitive as can be observed in Figures 6E vs. K.
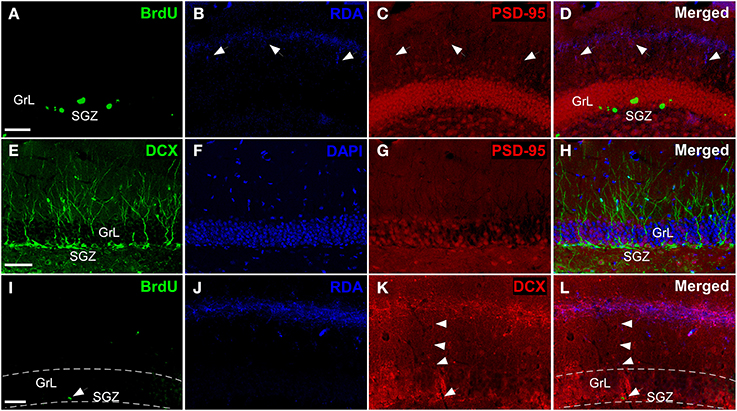
Figure 6. Triple labeling experiments in dentate gyrus. (A–D) Newly-born granule cells (green) were found in SGZ and arrows indicate connections between PSD-95+ cells (red) and rhodamine-labeled dextran-amine (RDA)-labeled fibers (blue) from cMEC. (E–H) Some of DCX+ cells (green), which are also localized in SGZ, express PSD-95 (red). (I–L) Arrowheads point out contacts between afferent RDA-labeled fibers (blue) from cMEC and BrdU (green)/DCX (red) double labeled cells. Calibration bar for (A–C) 50 μm.
In this sense, it is interesting to note that the length of the apical dendrites of DG granule cells -based on DCX expression and in agreement with previous studies (Torubarova et al., 1981)- is 95.5 μm ± 5.4 (s.e.m.) and 94.3 μm ± 6.9 (s.e.m.) in the caudoventral and rostrodorsal molecular layer of the DG, respectively. The outer and middle one-thirds of the molecular layer begin at 118.4 μm ± 9.8 (s.e.m.) and 74.2 μm ± 2.4 (s.e.m.) from the granule cell layer, respectively; which suggest that less 10% of the dendritic tree of DCX-positive granule cells reach the outer portion of the molecular layer.
Discussion
The data of the present work show that 3-week-old neurons born in the adult brain appear to receive inputs from distant structures. Newly-born granule and periglomerular cells in the OB, some of which co-express CR, could receive perisomatic inputs from axons of neurons located in the AON, OT or PIR (Figure 5). The centrifugal fibers, especially those originating in the AON and OT, appear to reach periglomerular cells (Figures 3C,G–I, 5A–D). Newly-born granule cells in the DG display apical dendrites over which axons from the MEC appear to make synapses in the middle one-third of the molecular layer. Although our results do not show it, the possibility that inputs from LEC reach the distal one-third of dendrites of newly-born cells cannot be discarded (Figure 7). Electron microscopy experiments have been carried out in order to further asses our results (see Supplementary Material), although results have been inconclusive due to technical difficulties.
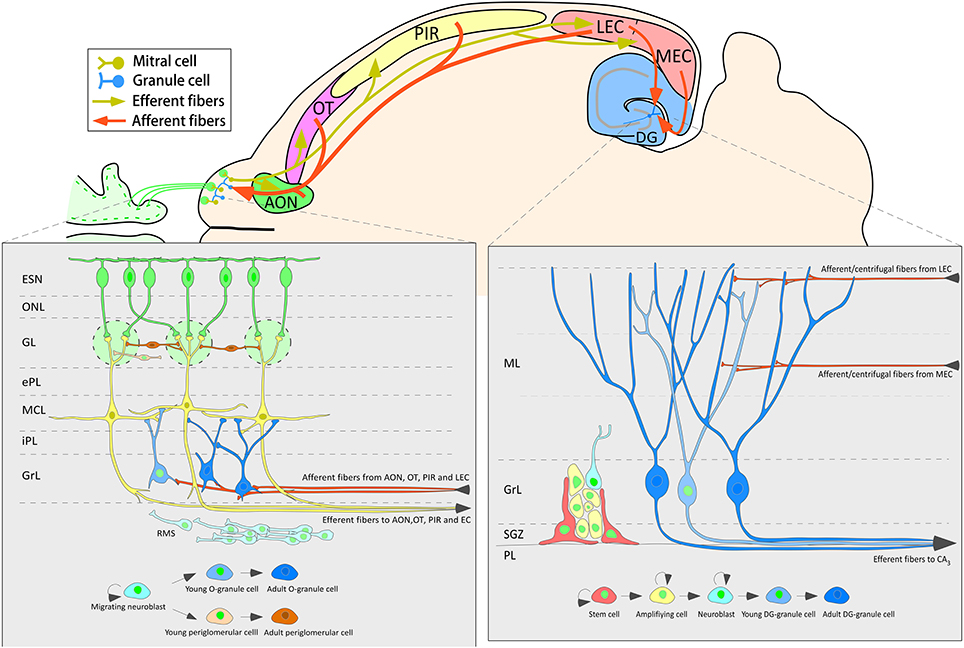
Figure 7. Schematic diagram of one horizontal section of the mouse brain summarizing the neurogenic processes and the main afferent connections to the olfactory bulb and dentate gyrus.
Adult-born Granule Cells in the Adult Olfactory Bulb and Dentate Gyrus
It has been estimated that 30.000 cells per day are generated bilaterally in the mouse SVZ (Lois and Alvarez-Buylla, 1994); and that about 650.000 neuroblasts are present in granule cell layer of the OB 30 days after H3thymidine administration (Petreanu and Alvarez-Buylla, 2002). This process seems quantitatively less relevant in the DG, since the SGZ daily produce approximately 9000 and 1200 neuroblasts per day in rats and mice, respectively (Kempermann et al., 1997; Christie and Cameron, 2006). This totalizes about 0.1% of all DG cells (West et al., 1991; Rapp and Gallagher, 1996; Cameron and McKay, 2001). Approximately 50% of new-born cells survive and 80% of those become fully mature neurons (Dayer et al., 2003). More than 650.000 new granule cells are present in the hippocampus of 3-months old rats (Snyder and Cameron, 2012). However, in humans, a recent study measuring 14C concentration in neuronal nuclei found a high turnover rate in the human hippocampus, 700 new neurons are added in each side per day, corresponding to an annual turnover of 1.75% of the neurons. These rates are comparable in middle-aged human and mice, suggesting adult hippocampal neurogenesis plays an important role in the maintenance of brain function (Spalding et al., 2013).
SVZ mainly provides GABAergic granule and periglomerular new interneurons to the OB. Granule cells are the main population of axonless, GABAergic interneurons in the OB. These interneurons appear to modulate the activity of mitral and tufted cells optimizing olfactory function by reducing overlap of odor representation in these projection cells (Mori and Shepherd, 1994; Yokoi et al., 1995; Isaacson and Strowbridge, 1998). Granule cells receive both GABAergic and glutamatergic synaptic inputs from cortical areas (Price and Powell, 1970) and OB intrinsic neurons (Bovetti et al., 2011). Granule cells receive two functionally different glutamatergic synaptic inputs: mitral cells excite granule cells mainly through distal dendrodendritic synapses; whereas proximal smaller dendritic spines receive contacts from cortical neurons. Thus, these feedback inputs mainly coming from the AON, OT, PIR, and LEC (Anaya-Martinez et al., 2006; Mohedano-Moriano et al., 2012) can gate dendrodendritic inhibition (Balu et al., 2007).
Dentate granule cells are one of the most intriguing cells in the central nervous system since they are glutamatergic neurons, but also express, inter alia, GABA, suggesting that they can act as inhibitory cells (Gutierrez, 2003; Gutierrez and Heinemann, 2006). These cells have been demonstrated to be involved in certain learning and memory tasks (Toga and Lothman, 1983; Shors et al., 2001; Snyder et al., 2005; Deng et al., 2009) and in emotional and spatial behaviors (Saxe et al., 2006; Clelland et al., 2009; Goodman et al., 2010).
Inputs onto Adult-Born Granule Neurons
Newly-born olfactory granule cells first receive GABAergic synaptic connections 3–7 days after birth, when the neuroblasts stop tangential migration and begin morphological maturation; and, 1 week thereafter, they begin to receive glutamatergic afferents (Belluzzi et al., 2003; Carleton et al., 2003; Panzanelli et al., 2009). Functional synapses at early stages of newly-born granule cells may establish a substrate for experience-depend regulation of adult neurogenesis and it can be important for long-term survival as data on blockade of glutamatergic activity have demonstrated (De Lima et al., 2004; Gascon et al., 2006; Panzanelli et al., 2009). Specifically, these centrifugal inputs may play an important role in maintenance and synaptic integration by regulation of dendritic growth of newly generated neurons in preexisting adult neuronal networks (Gascon et al., 2006; Mouret et al., 2009). However, knowledge about first synapses originating in neurons located in distant structures, including timing or specific origin is poor. In the present work, we have shown that olfactory afferents from AON, OT, and PIR reach and presumptively establish perisomatic contacts onto 21 day-old granule and periglomerular cells in the OB (Figure 5), even before they start to express specific olfactory population proteins.
Newly-born granule cells in the DG initially receive excitatory GABAergic inputs from local interneurons and this becomes inhibitory only once the glutamatergic extrinsic input from the EC has established, 2 weeks after birth (Overstreet Wadiche et al., 2005; Toni et al., 2007), showing most dendritic spines at 21–28 days after birth (Zhao et al., 2006). On the one hand, Li et al. (2012) have recently demonstrated that early GABAergic inputs may control the integration of maturing neurons to the adult hippocampal network. It has been shown that reduction of glutamatergic input implies loss of interneurons in other brain regions (De Lima et al., 2004). Many studies reveal the importance of entorhinal inputs to dentate granule cells. For example, unilateral or bilateral olfactory bulbectomy implies a rapid, transient increase in apoptosis in the dentate gyrus ipsilateral to the removed OB and lesions of the MEC significantly increase the number of dividing cells in the dentate gyrus in adult rats (Pope and Wilson, 2007). Data suggest that afferent input by NMDA receptor activation slows neuronal birth during adulthood (Cameron et al., 1995). Thereby, inputs seem to play a relevant role for cell survival, maturation and integration of new dentate granule cells once they reach maturity.
Dentate granule cells receive, through the perforant path, massive afferents from LEC and MEC (Steward, 1976; Witter, 2007). This pathway uses glutamate as primary neurotransmitter via NMDA receptors (White et al., 1977; Collingridge, 1989) but also presents a small GABAergic population (Germroth et al., 1989). Interestingly, neurons located in LEC send apical dendrites to layer I where they receive afferents from axons of bulbar mitral and tufted cells (Kosel et al., 1981; Martinez-Marcos and Halpern, 2006; Martinez-Marcos, 2009). Neurons located in layers II, V, and VI of LEC send axons to the outer one-third of the molecular layer of the DG; whereas the corresponding cells of MEC establish synapse in the middle one-third of this layer (Ramón Y Cajal, 1893; Hjorth-Simonsen, 1972; Hjorth-Simonsen and Jeune, 1972) (Figure 6). The dentate gyrus, therefore, receives olfactory information through the entorhinal cortex (Kosel et al., 1981) and the ventral subiculum and CA1 projects back to the OB (De La Rosa-Prieto et al., 2009b; Mohedano-Moriano et al., 2012). These data suggest that dentate granule cells could be also involved in olfactory processing, specifically in pattern separation for olfactory learning (Gilbert et al., 2001; Kesner et al., 2011), odor discrimination (Eichenbaum et al., 1988) and olfactory memory (Staubli et al., 1984).
Conflict of Interest Statement
The Associate Editor Jose Manuel Garcia-Verdugo and the Review Editor Francisco E. Olucha-Bordonau declare that, despite being affiliated to the same institution as authors Teresa Liberia and Carlos Crespo, the review process was handled objectively and no conflict of interest exists. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by the Spanish Ministry of Science and Innovation/FEDER (current Economy and Competitiveness Ministry) (BFU2010-15729). Dulce M. Arzate was supported by CONACyT and UNAM fellowships.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fnana.2015.00004/abstract
Abbreviations
AON, anterior olfactory nucleus; BrdU, bromodeoxyuridine; CR, calretinin; DCX, doublecortin; DG, dentate gyrus; EC, entorhinal cortex; GL, glomerular layer; GrL, granular cell layer; LEC, lateral entorhinal cortex; MEC, medial entorhinal cortex; OB, olfactory bulb; PB, phosphate buffer; PBS, phosphate buffered saline; PIR, piriform cortex; PSD-95: postsynaptic density-95; RDA, rhodamine-labeled dextran-amine; SGZ, subgranular zone; SVZ, subventricular zone; TBS, Tris buffered saline.
References
Aimone, J. B., Deng, W., and Gage, F. H. (2010). Adult neurogenesis: integrating theories and separating functions. Trends Cogn. Sci. 14, 325–337. doi: 10.1016/j.tics.2010.04.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Altman, J. (1962). Are new neurons formed in the brains of adult mammals? Science 13127–1128. doi: 10.1126/science.135.3509.1127
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Altman, J., and Das, G. D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319–335. doi: 10.1002/cne.901240303
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Alvarez-Buylla, A., and Garcia-Verdugo, J. M. (2002). Neurogenesis in adult subventricular zone. J. Neurosci. 22, 629–634. Available online at: http://www.jneurosci.org/content/22/3/629.long
Anaya-Martinez, V., Martinez-Marcos, A., Martinez-Fong, D., Aceves, J., and Erlij, D. (2006). Substantia nigra compacta neurons that innervate the reticular thalamic nucleus in the rat also project to striatum or globus pallidus: implications for abnormal motor behavior. Neuroscience 143, 477–486. doi: 10.1016/j.neuroscience.2006.08.033
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Balu, R., Pressler, R. T., and Strowbridge, B. W. (2007). Multiple modes of synaptic excitation of olfactory bulb granule cells. J. Neurosci. 27, 5621–5632. doi: 10.1523/JNEUROSCI.4630-06.2007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Belluzzi, O., Benedusi, M., Ackman, J., and Loturco, J. J. (2003). Electrophysiological differentiation of new neurons in the olfactory bulb. J. Neurosci. 23, 10411–10418. Available online at: http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=14614100
Bovetti, S., Gribaudo, S., Puche, A. C., De Marchis, S., and Fasolo, A. (2011). From progenitors to integrated neurons: role of neurotransmitters in adult olfactory neurogenesis. J. Chem. Neuroanat. 42, 304–316. doi: 10.1016/j.jchemneu.2011.05.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cameron, H. A., McEwen, B. S., and Gould, E. (1995). Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J. Neurosci. 15, 4687–4692.
Cameron, H. A., and McKay, R. D. (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 435, 406–417. doi: 10.1002/cne.1040
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Carlen, M., Cassidy, R. M., Brismar, H., Smith, G. A., Enquist, L. W., and Frisen, J. (2002). Functional integration of adult-born neurons. Curr. Biol. 12, 606–608. doi: 10.1016/S0960-9822(02)00771-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Carleton, A., Petreanu, L. T., Lansford, R., Alvarez-Buylla, A., and Lledo, P. M. (2003). Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 6, 507–518. doi: 10.1038/nn1048
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Christie, B. R., and Cameron, H. A. (2006). Neurogenesis in the adult hippocampus. Hippocampus 16, 199–207. doi: 10.1002/hipo.20151
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Clelland, C. D., Choi, M., Romberg, C., Clemenson, G. D. Jr., Fragniere, A., Tyers, P., et al. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213. doi: 10.1126/science.1173215
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Collingridge, G. L. (1989). “Syaptic function of N-methyl-D-aspartate receptors in the hippocampus,” in The Hippocampus: New Vistas, ed K. C. Chan-Palay V (New York, NY: Liss), 329–346.
Dayer, A. G., Ford, A. A., Cleaver, K. M., Yassaee, M., and Cameron, H. A. (2003). Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol. 460, 563–572. doi: 10.1002/cne.10675
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
De La Rosa-Prieto, C., Saiz-Sanchez, D., Ubeda-Banon, I., Argandona-Palacios, L., Garcia-Munozguren, S., and Martinez-Marcos, A. (2009a). Fate of marginal neuroblasts in the vomeronasal epithelium of adult mice. J. Comp. Neurol. 517, 723–736. doi: 10.1002/cne.22188
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
De La Rosa-Prieto, C., Ubeda-Banon, I., Mohedano-Moriano, A., Pro-Sistiaga, P., Saiz-Sanchez, D., Insausti, R., et al. (2009b). Subicular and CA1 hippocampal projections to the accessory olfactory bulb. Hippocampus 19, 124–129. doi: 10.1002/hipo.20495
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
De Lima, A. D., Opitz, T., and Voigt, T. (2004). Irreversible loss of a subpopulation of cortical interneurons in the absence of glutamatergic network activity. Eur. J. Neurosci. 19, 2931–2943. doi: 10.1111/j.0953-816X.2004.03403.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Deng, W., Saxe, M. D., Gallina, I. S., and Gage, F. H. (2009). Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 29, 13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eichenbaum, H., Fagan, A., Mathews, P., and Cohen, N. J. (1988). Hippocampal system dysfunction and odor discrimination learning in rats: impairment or facilitation depending on representational demands. Behav. Neurosci. 102, 331–339. doi: 10.1037/0735-7044.102.3.331
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Franklin, K. B. J., and Paxinos, G. (2008). The Mouse Brain in Stereotaxic Coordinates. New York, NY: Academic Press.
Gascon, E., Dayer, A. G., Sauvain, M. O., Potter, G., Jenny, B., De Roo, M., et al. (2006). GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J. Neurosci. 26, 12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Germroth, P., Schwerdtfeger, W. K., and Buhl, E. H. (1989). GABAergic neurons in the entorhinal cortex project to the hippocampus. Brain Res. 494, 187–192. doi: 10.1016/0006-8993(89)90162-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gilbert, P. E., Kesner, R. P., and Lee, I. (2001). Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus 11, 626–636. doi: 10.1002/hipo.1077
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Goodman, T., Trouche, S., Massou, I., Verret, L., Zerwas, M., Roullet, P., et al. (2010). Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience 171, 769–778. doi: 10.1016/j.neuroscience.2010.09.047
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gutierrez, R. (2003). The GABAergic phenotype of the “glutamatergic” granule cells of the dentate gyrus. Prog. Neurobiol. 71, 337–358. doi: 10.1016/j.pneurobio.2003.11.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gutierrez, R., and Heinemann, U. (2006). Co-existence of GABA and Glu in the hippocampal granule cells: implications for epilepsy. Curr. Top. Med. Chem. 6, 975–978. doi: 10.2174/156802606777323692
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hjorth-Simonsen, A. (1972). Projection of the lateral part of the entorhinal area to the hippocampus and fascia dentata. J. Comp. Neurol. 146, 219–232. doi: 10.1002/cne.901460206
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hjorth-Simonsen, A., and Jeune, B. (1972). Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J. Comp. Neurol. 144, 215–232. doi: 10.1002/cne.901440206
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Isaacson, J. S., and Strowbridge, B. W. (1998). Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron 20, 749–761. doi: 10.1016/S0896-6273(00)81013-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kaneko, N., Marin, O., Koike, M., Hirota, Y., Uchiyama, Y., Wu, J. Y., et al. (2010). New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron 67, 213–223. doi: 10.1016/j.neuron.2010.06.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kempermann, G., Kuhn, H. G., and Gage, F. H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495. doi: 10.1038/386493a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kesner, R. P., Hunsaker, M. R., and Ziegler, W. (2011). The role of the dorsal and ventral hippocampus in olfactory working memory. Neurobiol. Learn. Mem. 96, 361–366. doi: 10.1016/j.nlm.2011.06.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kosel, K. C., Van Hoesen, G. W., and West, J. R. (1981). Olfactory bulb projections to the parahippocampal area of the rat. J. Comp. Neurol. 198, 467–482. doi: 10.1002/cne.901980307
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lepousez, G., Valley, M. T., and Lledo, P. M. (2013). The impact of adult neurogenesis on olfactory bulb circuits and computations. Annu. Rev. Physiol. 75, 339–363. doi: 10.1146/annurev-physiol-030212-183731
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Li, Y., Aimone, J. B., Xu, X., Callaway, E. M., and Gage, F. H. (2012). Development of GABAergic inputs controls the contribution of maturing neurons to the adult hippocampal network. Proc. Natl. Acad. Sci. U.S.A. 109, 4290–4295. doi: 10.1073/pnas.1120754109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lledo, P. M., Alonso, M., and Grubb, M. S. (2006). Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 7, 179–193. doi: 10.1038/nrn1867
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lledo, P. M., and Saghatelyan, A. (2005). Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 28, 248–254. doi: 10.1016/j.tins.2005.03.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lois, C., and Alvarez-Buylla, A. (1994). Long-distance neuronal migration in the adult mammalian brain. Science 264, 1145–1148. doi: 10.1126/science.8178174
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Luskin, M. B. (1993). Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11, 173–189. doi: 10.1016/0896-6273(93)90281-U
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martinez-Marcos, A. (2009). On the organization of olfactory and vomeronasal cortices. Prog. Neurobiol. 87, 21–30. doi: 10.1016/j.pneurobio.2008.09.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martinez-Marcos, A., and Halpern, M. (2006). Efferent connections of the main olfactory bulb in the opossum (Monodelphis domestica): a characterization of the olfactory entorhinal cortex in a marsupial. Neurosci. Lett. 395, 51–56. doi: 10.1016/j.neulet.2005.10.052
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martinez-Marcos, A., Jia, C., Quan, W., and Halpern, M. (2005). Neurogenesis, migration, and apoptosis in the vomeronasal epithelium of adult mice. J. Neurobiol. 63, 173–187. doi: 10.1002/neu.20128
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mohedano-Moriano, A., De La Rosa-Prieto, C., Saiz-Sanchez, D., Ubeda-Banon, I., Pro-Sistiaga, P., De Moya-Pinilla, M., et al. (2012). Centrifugal telencephalic afferent connections to the main and accessory olfactory bulbs. Front. Neuroanat. 6:19. doi: 10.3389/fnana.2012.00019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mori, K., and Shepherd, G. M. (1994). Emerging principles of molecular signal processing by mitral/tufted cells in the olfactory bulb. Semin. Cell Biol. 5, 65–74. doi: 10.1006/scel.1994.1009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mouret, A., Murray, K., and Lledo, P. M. (2009). Centrifugal drive onto local inhibitory interneurons of the olfactory bulb. Ann. N.Y. Acad. Sci. 1170, 239–254. doi: 10.1111/j.1749-6632.2009.03913.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nguyen-Ba-Charvet, K. T., Picard-Riera, N., Tessier-Lavigne, M., Baron-Van Evercooren, A., Sotelo, C., and Chedotal, A. (2004). Multiple roles for slits in the control of cell migration in the rostral migratory stream. J. Neurosci. 24, 1497–1506. doi: 10.1523/JNEUROSCI.4729-03.2004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Overstreet Wadiche, L., Bromberg, D. A., Bensen, A. L., and Westbrook, G. L. (2005). GABAergic signaling to newborn neurons in dentate gyrus. J. Neurophysiol. 94, 4528–4532. doi: 10.1152/jn.00633.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pallotto, M., and Deprez, F. (2014). Regulation of adult neurogenesis by GABAergic transmission: signaling beyond GABAA-receptors. Front. Cell. Neurosci. 8:166. doi: 10.3389/fncel.2014.00166
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Panzanelli, P., Bardy, C., Nissant, A., Pallotto, M., Sassoe-Pognetto, M., Lledo, P. M., et al. (2009). Early synapse formation in developing interneurons of the adult olfactory bulb. J. Neurosci. 29, 15039–15052. doi: 10.1523/JNEUROSCI.3034-09.2009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Petreanu, L., and Alvarez-Buylla, A. (2002). Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 22, 6106–6113.
Pope, K., and Wilson, D. A. (2007). Olfactory system modulation of hippocampal cell death. Neurosci. Lett. 422, 13–17. doi: 10.1016/j.neulet.2007.05.041
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Price, J. L., and Powell, T. P. (1970). An experimental study of the origin and the course of the centrifugal fibres to the olfactory bulb in the rat. J. Anat. 107, 215–237.
Pro-Sistiaga, P., Mohedano-Moriano, A., Ubeda-Banon, I., Del Mar Arroyo-Jimenez, M., Marcos, P., Artacho-Perula, E., et al. (2007). Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J. Comp. Neurol. 504, 346–362. doi: 10.1002/cne.21455
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ramón Y Cajal, S. (1893). Estructura del asta de Ammon y fascia dentata. Ann. Soc. Esp. Hist. Nat. 22, 53–114.
Rapp, P. R., and Gallagher, M. (1996). Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. U.S.A. 93, 9926–9930. doi: 10.1073/pnas.93.18.9926
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sahay, A., Wilson, D. A., and Hen, R. (2011). Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588. doi: 10.1016/j.neuron.2011.05.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sakamoto, M., Kageyama, R., and Imayoshi, I. (2014). The functional significance of newly born neurons integrated into olfactory bulb circuits. Front. Neurosci. 8:121. doi: 10.3389/fnins.2014.00121
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Saxe, M. D., Battaglia, F., Wang, J. W., Malleret, G., David, D. J., Monckton, J. E., et al. (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 103, 17501–17506. doi: 10.1073/pnas.0607207103
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T., and Gould, E. (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–376. doi: 10.1038/35066584
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Snyder, J. S., and Cameron, H. A. (2012). Could adult hippocampal neurogenesis be relevant for human behavior? Behav. Brain Res. 227, 384–390. doi: 10.1016/j.bbr.2011.06.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Snyder, J. S., Hong, N. S., McDonald, R. J., and Wojtowicz, J. M. (2005). A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–852. doi: 10.1016/j.neuroscience.2004.10.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spalding, K. L., Bergmann, O., Alkass, K., Bernard, S., Salehpour, M., Huttner, H. B., et al. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227. doi: 10.1016/j.cell.2013.05.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Staubli, U., Ivy, G., and Lynch, G. (1984). Hippocampal denervation causes rapid forgetting of olfactory information in rats. Proc. Natl. Acad. Sci. U.S.A. 81, 5885–5887. doi: 10.1073/pnas.81.18.5885
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Steward, O. (1976). Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J. Comp. Neurol. 167, 285–314. doi: 10.1002/cne.901670303
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tashiro, A., Sandler, V. M., Toni, N., Zhao, C., and Gage, F. H. (2006). NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442, 929–933. doi: 10.1038/nature05028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Toga, A. W., and Lothman, E. W. (1983). Learning deficits after lesions of dentate gyrus granule cells. Exp. Neurol. 82, 192–202. doi: 10.1016/0014-4886(83)90254-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Toni, N., Teng, E. M., Bushong, E. A., Aimone, J. B., Zhao, C., Consiglio, A., et al. (2007). Synapse formation on neurons born in the adult hippocampus. Nat. Neurosci. 10, 727–734. doi: 10.1038/nn1908
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Torubarova, N. A., Kosel, I. V., Savinskij, A. K., Cistopolskij, A. S., Jesimova, S. S., Reznik, I. V., et al. (1981). [Retrospective analysis of 61 cases of acquired juvenile hypoplastic anemia. Factors determining the prognosis]. Folia Haematol. Int. Mag. Klin. Morphol. Blutforsch. 108, 778–785.
Van Der Borght, K., Mulder, J., Keijser, J. N., Eggen, B. J., Luiten, P. G., and Van Der Zee, E. A. (2005). Input from the medial septum regulates adult hippocampal neurogenesis. Brain Res. Bull. 67, 117–125. doi: 10.1016/j.brainresbull.2005.06.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Praag, H., Schinder, A. F., Christie, B. R., Toni, N., Palmer, T. D., and Gage, F. H. (2002). Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034. doi: 10.1038/4151030a
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
West, M. J., Slomianka, L., and Gundersen, H. J. (1991). Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482–497. doi: 10.1002/ar.1092310411
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
White, W. F., Nadler, J. V., Hamberger, A., Cotman, C. W., and Cummins, J. T. (1977). Glutamate as transmitter of hippocampal perforant path. Nature 270, 356–357. doi: 10.1038/270356a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Whitman, M. C., Fan, W., Rela, L., Rodriguez-Gil, D. J., and Greer, C. A. (2009). Blood vessels form a migratory scaffold in the rostral migratory stream. J. Comp. Neurol. 516, 94–104. doi: 10.1002/cne.22093
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Whitman, M. C., and Greer, C. A. (2009). Adult neurogenesis and the olfactory system. Prog. Neurobiol. 89, 162–175. doi: 10.1016/j.pneurobio.2009.07.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Witter, M. P. (2007). The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog. Brain Res. 163, 43–61. doi: 10.1016/S0079-6123(07)63003-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yokoi, M., Mori, K., and Nakanishi, S. (1995). Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc. Natl. Acad. Sci. U.S.A. 92, 3371–3375. doi: 10.1073/pnas.92.8.3371
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhao, C., Teng, E. M., Summers, R. G. Jr., Ming, G. L., and Gage, F. H. (2006). Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 26, 3–11. doi: 10.1523/JNEUROSCI.3648-05.2006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: adult neurogenesis, hippocampus, memory, olfaction, tract-tracing, synapse
Citation: De La Rosa Prieto C, De Moya Pinilla M, Saiz-Sanchez D, Ubeda-banon I, Arzate DM, Flores-Cuadrado A, Liberia T, Crespo C and Martinez-Marcos A (2015) Olfactory and cortical projections to bulbar and hippocampal adult-born neurons. Front. Neuroanat. 9:4. doi: 10.3389/fnana.2015.00004
Received: 28 July 2014; Accepted: 09 January 2015;
Published online: 02 February 2015.
Edited by:
Jose Manuel Garcia-Verdugo, University of Valencia, SpainReviewed by:
Antonio Pereira, Federal University of Rio Grande do Norte, BrazilFrancisco E. Olucha-Bordonau, University of Valencia, Spain
Copyright © 2015 De La Rosa Prieto, De Moya Pinilla, Saiz-Sanchez, Ubeda-banon, Arzate, Flores-Cuadrado, Liberia, Crespo and Martinez-Marcos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alino Martinez-Marcos, Facultad de Medicina de Ciudad Real, Universidad de Castilla-La Mancha, Avda. de Moledores s/n, 13071 Ciudad Real, Spain e-mail: alino.martinez@uclm.es
 Carlos De La Rosa-Prieto
Carlos De La Rosa-Prieto Miguel De Moya-Pinilla
Miguel De Moya-Pinilla Daniel Saiz-Sanchez
Daniel Saiz-Sanchez Isabel Ubeda-banon
Isabel Ubeda-banon Dulce M. Arzate
Dulce M. Arzate Alicia Flores-Cuadrado1
Alicia Flores-Cuadrado1  Carlos Crespo
Carlos Crespo Alino Martinez-Marcos
Alino Martinez-Marcos