- 1Department of Microbiology, University of Massachusetts, Amherst, MA, USA
- 2Nordic Center for Earth Evolution, Department of Biology, University of Southern Denmark, Odense, Denmark
Electrodes are unnatural electron acceptors, and it is yet unknown how some Geobacter species evolved to use electrodes as terminal electron acceptors. Analysis of different Geobacter species revealed that they varied in their capacity for current production. Geobacter metallireducens and G. hydrogenophilus generated high current densities (ca. 0.2 mA/cm2), comparable to G. sulfurreducens. G. bremensis, G. chapellei, G. humireducens, and G. uraniireducens, produced much lower currents (ca. 0.05 mA/cm2) and G. bemidjiensis was previously found to not produce current. There was no correspondence between the effectiveness of current generation and Fe(III) oxide reduction rates. Some high-current-density strains (G. metallireducens and G. hydrogenophilus) reduced Fe(III)-oxides as fast as some low-current-density strains (G. bremensis, G. humireducens, and G. uraniireducens) whereas other low-current-density strains (G. bemidjiensis and G. chapellei) reduced Fe(III) oxide as slowly as G. sulfurreducens, a high-current-density strain. However, there was a correspondence between the ability to produce higher currents and the ability to grow syntrophically. G. hydrogenophilus was found to grow in co-culture with Methanosarcina barkeri, which is capable of direct interspecies electron transfer (DIET), but not with Methanospirillum hungatei capable only of H2 or formate transfer. Conductive granular activated carbon (GAC) stimulated metabolism of the G. hydrogenophilus – M. barkeri co-culture, consistent with electron exchange via DIET. These findings, coupled with the previous finding that G. metallireducens and G. sulfurreducens are also capable of DIET, suggest that evolution to optimize DIET has fortuitously conferred the capability for high-density current production to some Geobacter species.
Introduction
Geobacter species are among the most effective microorganisms for harvesting electrical current from organic compounds (Call and Logan, 2011; Lovley et al., 2011; Kumar et al., 2015). However, the electrodes that serve as the electron acceptors in current production are not found in the soils and sediments that are the natural habitat of Geobacter species. Therefore, the selective pressure to optimize the reduction of other extracellular electron acceptors, which Geobacter species naturally utilize, may have fortuitously lead to the superior ability of Geobacter species to produce high current densities. If the natural analog for electrodes could be identified this could aid in understanding of the mechanisms for electron transfer to electrodes as well as guide strategies to improve the current production capabilities of Geobacter species. Two potential natural analogs are poorly crystalline insoluble Fe(III) oxides and direct interspecies electron transfer (DIET) partners.
The primary electron acceptor for Geobacter species in many soils and sediments is poorly crystalline insoluble Fe(III) oxides (Lovley et al., 2011). Electrodes and Fe(III) oxides are both extracellular electron acceptors and therefore it is possible that the evolution of Geobacter species to excel at Fe(III) oxide reduction also yielded characteristics for effective current production. However, there are important differences in the properties of electrodes and Fe(III) oxides. For example, a current-harvesting electrode provides a long-term, stable electron sink for Geobacter respiration whereas an Fe(III) oxide particle has a limited, finite capacity to accept electrons. Furthermore, electrodes are typically much larger than Geobacter cells, whereas most Fe(III) oxide minerals in soils, as well as the poorly crystalline Fe(III) oxides typically employed in culture medium (Lovley and Phillips, 1988), are much smaller than the cells. Therefore, Geobacter species cannot form biofilms on Fe(III) oxides and motile cells that can search for new Fe(III) oxide sources appear to have a selective advantage in Fe(III) oxide reduction (Childers et al., 2002; Tremblay et al., 2012; Ueki et al., 2012). This is evident in subsurface environments in which Geobacter species are actively reducing Fe(III) oxides, where the cells are readily recovered in the groundwater (Anderson et al., 2003; Holmes et al., 2007, 2015). In contrast, Geobacter species oxidizing organic compounds with an electrode as the electron acceptor attach to the electrode surface and can form biofilms many cell layers thick (Reguera et al., 2006; Nevin et al., 2008; Richter et al., 2008; Franks et al., 2012).
A more appropriate natural analog for Geobacter electrode biofilms might be the cell aggregations established during DIET. When Geobacter metallireducens and G. sulfurreducens were grown in co-culture in a medium, which contained an electron donor that only G. metallireducens could metabolize (ethanol) and an electron acceptor that only G. sulfurreducens could reduce (fumarate), the two species formed large (1 mm diameter) aggregates (Summers et al., 2010; Shrestha et al., 2013a). The aggregates were electrically conductive (Summers et al., 2010), similar to anode biofilms (Malvankar et al., 2011). Conductive Geobacter-rich aggregates have been noted in anaerobic digesters converting organic wastes to methane (Morita et al., 2011; Shrestha et al., 2014) and defined co-cultures of G. metallireducens and either Methanosaeta (Rotaru et al., 2014b) or Methanosarcina (Rotaru et al., 2014a) species form visible aggregates to share electrons via DIET. The abundance of Geobacter species in some methanogenic soils and sediments (Hori et al., 2007; Kato et al., 2012; Xu et al., 2013) as well as the ability of conductive minerals to simultaneously enhance the growth of Geobacter species and methane production (Kato et al., 2012; Liu et al., 2012; Chen et al., 2014a,b; Cruz Viggi et al., 2014; Li et al., 2014; Rotaru et al., 2014a; Shrestha and Rotaru, 2014) suggests that co-aggregation of Geobacter species and methanogens may be a common phenomenon in these methanogenic environments as well.
Although the details of long-range electron transfer through current-producing biofilms and aggregates involved in DIET are still being elucidated, the electrically conductive pili of Geobacter species, known as microbial nanowires, are central to both processes as well as for Fe(III) oxide reduction (Reguera et al., 2005, 2006; Nevin et al., 2009; Tremblay et al., 2012; Vargas et al., 2013; Lovley and Malvankar, 2015). Studies of G. sulfurreducens pili have revealed that pili possess metallic-like conductivity (Malvankar et al., 2011), which can be attributed to overlapping π–π orbitals of aromatic amino acids (Vargas et al., 2013; Lovley and Malvankar, 2015). Genetically eliminating the capacity for pili production (Reguera et al., 2005; Tremblay et al., 2012) or diminishing pili conductivity (Vargas et al., 2013; Liu et al., 2014) severely reduces Fe(III) oxide reduction and current production, whereas increasing expression of pili yields higher currents (Yi et al., 2009; Leang et al., 2013). In a similar manner, co-culture aggregates sharing electrons via DIET could not be established with a strain of G. metallireducens that could not produce pili (Shrestha et al., 2013b; Rotaru et al., 2014a,b).
Other outer-surface proteins, including c-type cytochromes, are also required for extracellular electron transfer to Fe(III) oxides, electrodes, or syntrophic partners (Lovley et al., 2011). However, the lack of a full understanding of how all these components interact, especially in biofilms and aggregates, has made it as yet impossible to make direct comparison of the mechanisms for electron transfer to Fe(III) oxides, other cells, and electrodes.
Previous studies on current production, Fe(III) oxide reduction, and DIET by Geobacter species have primarily focused on G. sulfurreducens, because it is closely related to the Geobacter species that often predominate in current-harvesting biofilms and because it can readily be genetically manipulated (Lovley et al., 2011; Mahadevan et al., 2011). Therefore, in order to gain insight into whether Fe(III) oxide reduction or DIET might be a better natural analog for electron transfer to electrodes, we compared the ability of a diversity of other Geobacter species to produce current, reduce Fe(III) oxide, and participate in DIET.
Materials and Methods
Source of Organisms and Routine Cultivation
All Geobacter species were from our laboratory culture collection. Methanosarcina barkeri (DSM 800) was purchased from the German Culture Collection.
Cultivation was performed using strict anaerobic cultivation protocols (Balch et al., 1979). With the exception of cultivation with Fe(III) oxide as the electron acceptor (see below), all media were boiled and then cooled under N2:CO2 (80:20) to remove dissolved oxygen. The medium was dispensed in culture tubes and sterilized under a N2:CO2 (80:20) atmosphere. Substrates and vitamins were added from anaerobic, filtered sterilized stocks after the medium was autoclaved.
For routine cultivation G. metallireducens, G. humireducens, G. hydrogenophilus, G. bremensis, G. bemidjiensis, and G. sulfurreducens were provided with 50 mM Fe(III) citrate as electron acceptor, as previously described (Lovley and Phillips, 1988; Coates et al., 2001; Straub and Buchholz-Cleven, 2001; Nevin et al., 2005; Tremblay et al., 2012), with the exception that Fe(III) citrate was added from an anaerobic, sterile stock after the medium was sterilized. G. uraniireducens (Shelobolina et al., 2008), and G. chapellei (Coates et al., 2001), which can not use Fe(III) citrate as an electron acceptor, were provided with 40 mM fumarate as electron acceptor.
Methanosarcina barkeri was cultured anaerobically with 30 or 40 mM acetate as substrate on a modified DSMZ medium 120, as previously described (Rotaru et al., 2014a).
Current Production
The capacity for current production was determined in flow-through, two-chambered H-cell systems with graphite stick anodes (65 cm2) poised at 300 mV, with a continuous supply of fresh acetate (10 mM) medium, as previously described (Nevin et al., 2009). Briefly, Geobacter species other than G. metallireducens, were pre-grown in fumarate (40 mM) and acetate (10 mM) media in the anode chamber and then the medium was replaced with medium containing only acetate (10 mM). For G. metallireducens, which does not grow on fumarate, cells were pre-grown in media containing Fe(III) citrate (55 mM) and acetate (10 mM), harvested by centrifugation, resuspended in bicarbonate buffer (30 mM), and inoculated into the anode chamber containing acetate (10 mM) medium.
Fe(III) Oxide Reduction
All Geobacter species were adapted to grow effectively on ethanol or lactate prior to Fe(III)-oxide reduction tests for at least three transfers. All cultures grew overnight on these substrates if the electron acceptor was Fe(III)-citrate. Cultures were grown with poorly crystalline Fe(III) oxide (100 mmol/liter) as the electron acceptor as previously described (Lovley and Phillips, 1988) with the exception that ethanol (20 mM) was the electron donor for all cultures expect G. sulfurreducens which is unable to utilize ethanol and was provided with lactate (10 mM) as the electron donor.
Co-Cultivation with M. barkeri
Prior to growth in co-cultures the Geobacter species were adapted to grow using ethanol for at least three transfers. We choose ethanol because it is the only known DIET-syntrophic substrate (Rotaru et al., 2014a,b).
Co-cultures of Geobacter species and M. barkeri were initiated in medium with ethanol (20 mM) as the electron donor and carbon dioxide as the only potential electron acceptor, as previously described (Rotaru et al., 2014a). Co-cultures were initiated with a 5% inoculum of each partner organism grown to mid- or late-logarithmic, as previously described (Rotaru et al., 2014a).
In order to evaluate the impact of granular activated carbon (GAC) on co-culture growth 0.1 g of GAC was added to the culture tubes along with 0.2 ml ultrapure water, sealed and sterilized at 121°C, under a N2:CO2 atmosphere for 1 h. Then 9 ml of medium and 5% inoculum of each partner organism were added to the anoxic, sterile tubes.
Analytical Measurements
Samples for metabolite analyses were retrieved with hypodermic needles and syringes flushed with N2-CO2. For methane analysis, headspace samples (0.5 ml) were retrieved with a gas tight syringe and injected immediately on a Shimadzu gas chromatograph as previously described (Rotaru et al., 2014a). For ethanol and short chain volatile fatty acid analysis, 0.2 ml culture of medium was sampled aseptically and anaerobically with sterile pre-flushed syringes. Ethanol was measured on a gas chromatograph equipped with a flame ionization detector as previously described (Rotaru et al., 2014a). Short chain fatty acids were quantified with high performance liquid chromatography using a fast acid column (Rotaru et al., 2014a).
In cultures grown with Fe(III) oxide as terminal electron acceptor, Fe(II) was monitored with the ferrozine assay as previously described (Lovley and Phillips, 1987; Anderson and Lovley, 1999).
Results and Discussions
Current-Producing Capacity of Diverse Geobacter Species
In order to evaluate possible links between the capacity for current production and either Fe(III) oxide reduction or DIET, each of these processes were studied in seven species other than G. sulfurreducens. G. metallireducens (Figure 1) and G. hydrogenophilus (Figure 1) both produced currents (ca. 0.2 mA/cm2) comparable to those previously reported for G. sulfurreducens (Nevin et al., 2009). However, all the other strains tested generated much lower (ca. 0.05 mA/cm2) maximum currents (Figure 1). Furthermore, G. bemidjiensis was unable to produce current (Nevin et al., 2005). These results demonstrate that not all Geobacter species are highly effective current producers. The best current producers were G. sulfurreducens, G. metallireducens, and G. hydrogenophilus, which are closely related (Lovley et al., 2011). This suggests that common physiological factors specific to the evolution of these species confer the capacity for exceptional current production. These results may also explain why many studies have found that Geobacter species closely related to G. sulfurreducens predominate on electrodes harvesting electricity from mixed microbial communities (Lovley et al., 2011; Yates et al., 2012).
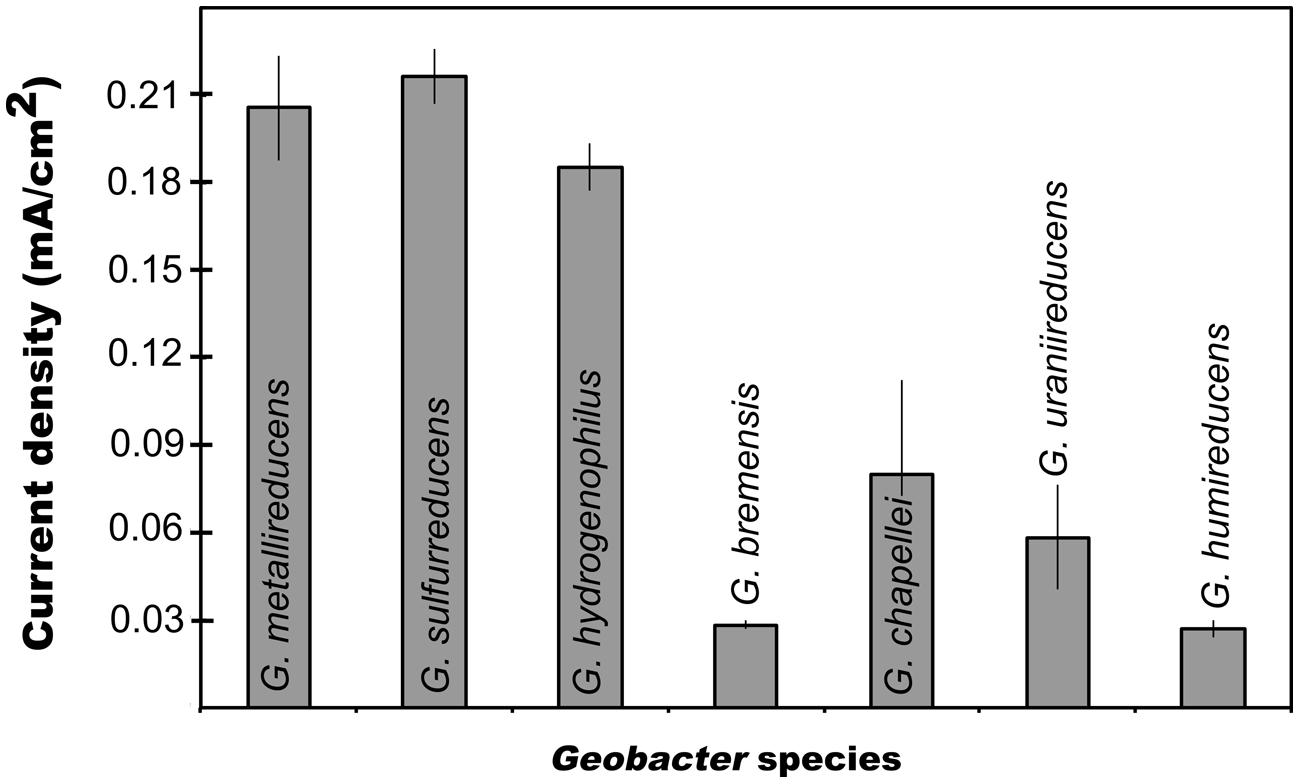
FIGURE 1. Maximum current production for different Geobacter species. Results are means of duplicate determinations.
Fe(III) Oxide Reduction Capabilities
In order to determine whether there was any correspondence between the effectiveness of current production and the ability to reduce insoluble Fe(III) oxides, each of the Geobacter species was grown in medium with insoluble Fe(III) oxide as the sole electron acceptor. The inoculum for each Geobacter strain grew rapidly overnight in their medium with soluble electron acceptor, but there were marked differences in the rate of metabolism in Fe(III) oxide medium. G. chapellei and G. bemidjiensis, two species that produced low currents, slowly reduced Fe(III) oxide slowly with maximum rates of Fe(II) production of 0.02 ± 0.06 and 0.04 ± 0.07 mM Fe(II) per hour, respectively (Figure 2). However, three other species with low current outputs, G. bremensis, G. humireducens, and G. uraniireducens, were highly effective Fe(III) oxide reducers with maximum Fe(III) oxide reduction rates of 0.44 ± 0.12, 0.16 ± 0.05, and 0.23 ± 0.03 mM Fe(II) per hour, respectively (Figure 2). G. metallireducens and G. hydrogenophilus, which produced high current densities were also very proficient insoluble Fe-oxide reducers (Figure 2) with maximum Fe(III) oxide reduction rates of 0.15 ± 0.02, and 0.33 ± 0.16 mM Fe(II) per hour, respectively (Figure 2). In contrast G. sulfurreducens, which is also highly effective in current production (Nevin et al., 2009), slowly reduced Fe(III) oxide [0.02 ± 0.06 mM Fe(II) per hour]. These results demonstrated that there is no correspondence between the capacities for Fe(III) oxide reduction and current production among these eight Geobacter species.
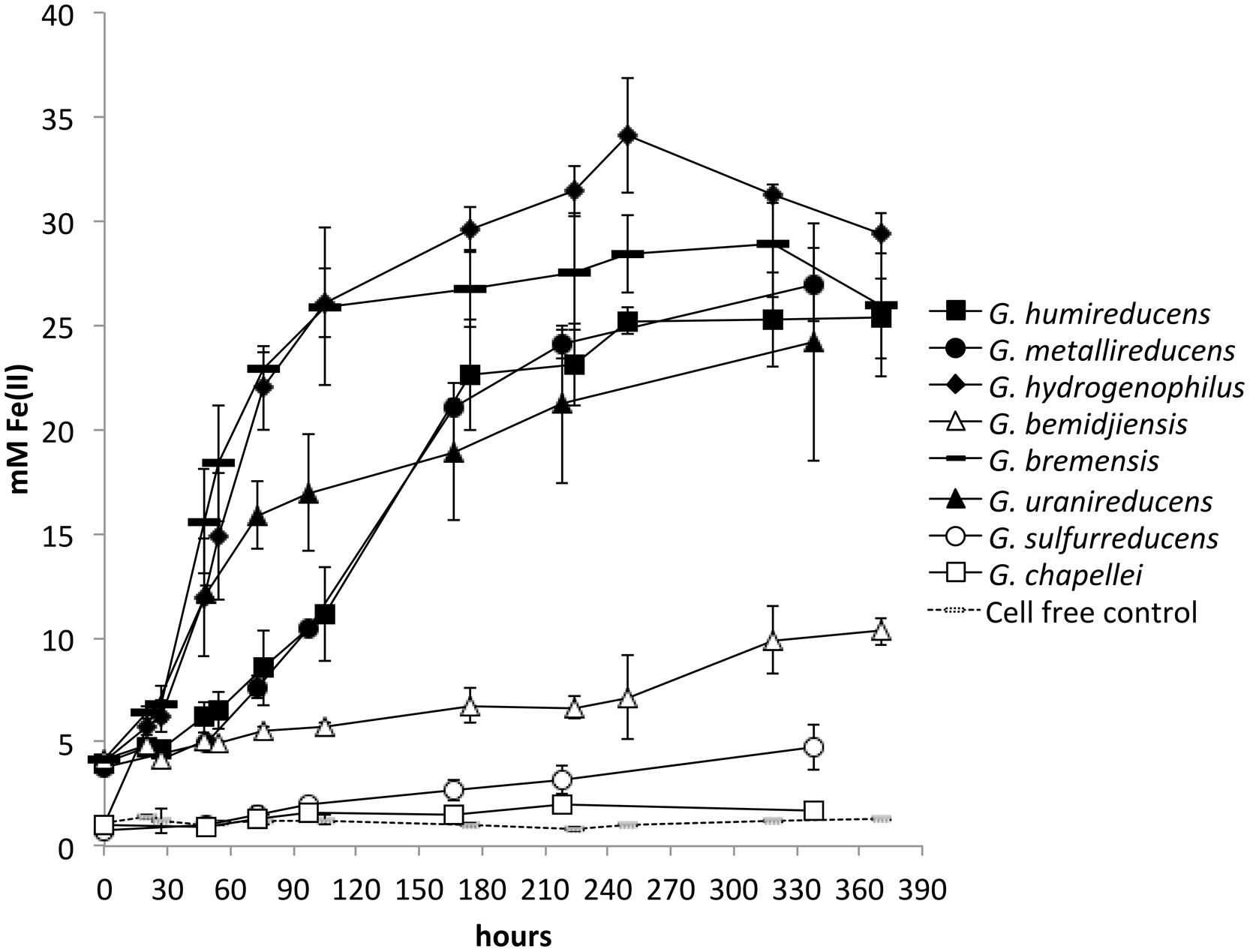
FIGURE 2. Fe(II) production from insoluble Fe(III) oxides by different Geobacter species. All species except for Geobacter sulfurreducens were provided with ethanol as electron donor. G. sulfurreducens cannot grow on ethanol, and was provided with 10 mM lactate as electron donor. Results are the mean and SD of triplicate cultures for each species.
The reason for the between species differences in rates of Fe(III) oxide reduction require further investigation, but may be related to distinct selective pressures in the diverse environments from which these Geobacter species have been isolated. Furthermore, the different enrichment and isolation procedures by which many of these pure cultures were obtained may have selected for unique physiological characteristics which are reflected in the range of Fe(III) oxide reduction rates observed. One indication of this possibility is the lack of between species conservation in the c-type cytochromes likely to be involved in extracellular electron transfer (Butler et al., 2010).
Syntrophic Growth with M. barkeri
Previous studies have demonstrated that both G. metallireducens and G. sulfurreducens, which produce high current densities, are also capable of DIET. G. sulfurreducens directly accepted electrons from G. metallireducens (Summers et al., 2010). G. metallireducens was capable of serving as the electron-donating partner in DIET with either G. sulfurreducens (Summers et al., 2010), Methanosaeta harundinacea (Rotaru et al., 2014b) or M. barkeri (Rotaru et al., 2014a) as the electron-accepting partner.
In order to determine if any other Geobacter species might function in a similar manner, co-cultures were initiated with M. barkeri. Of the Geobacter species evaluated, only G. hydrogenophilus successfully established co-cultures with M. barkeri (Figures 3 and 4), whereas G. bemidjiensis, G. bremensis, G. chapellei, G. humireducens, and G. uraniireducens did not (Figure 4).
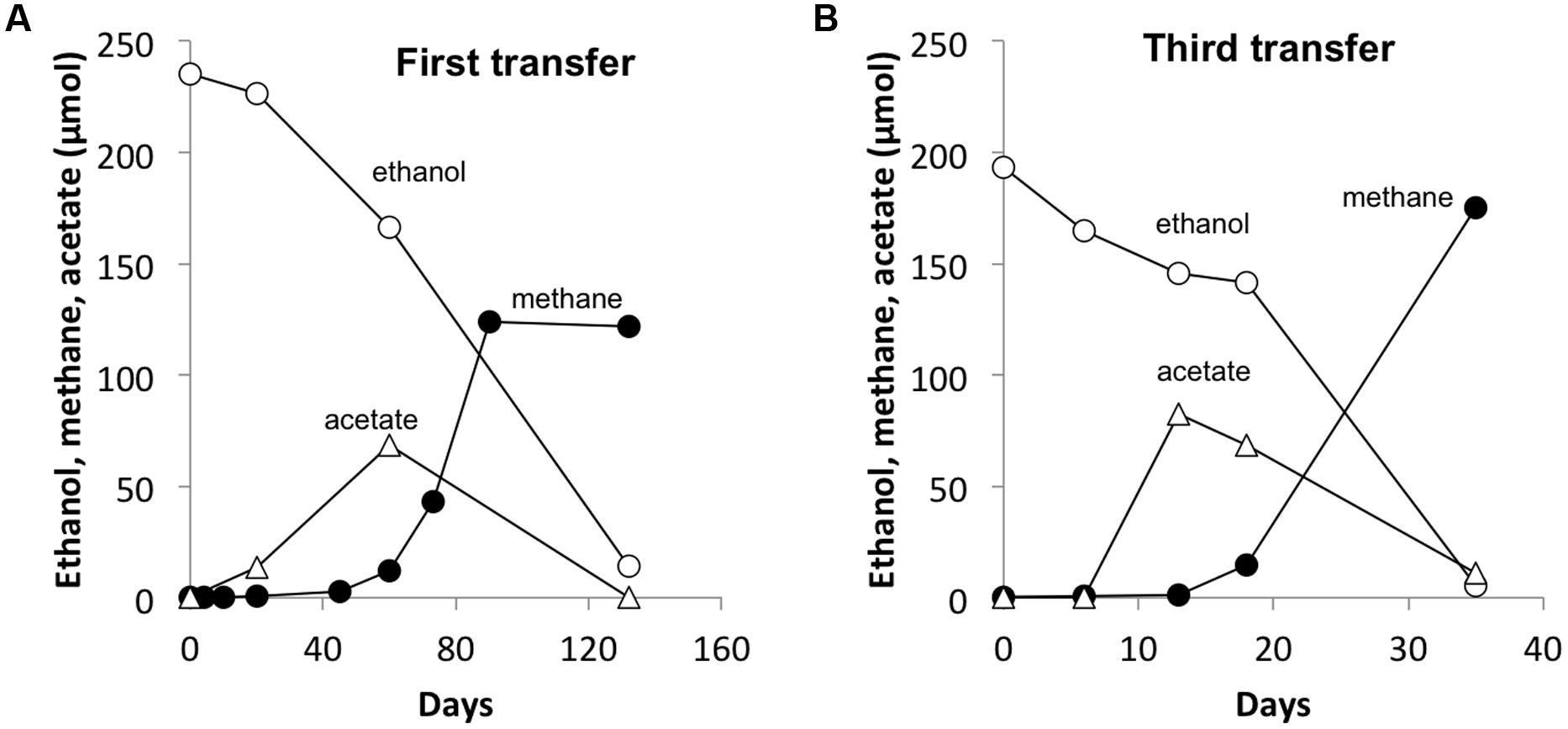
FIGURE 3. A representative co-culture of G. hydrogenophilus – Methanosarcina barkeri during the first (A) and the third transfer (B). Notice the four-time decrease in time scale from the first to the third transfer. Values are from single incubations. See Supplementary Materials for all three replicate incubations.
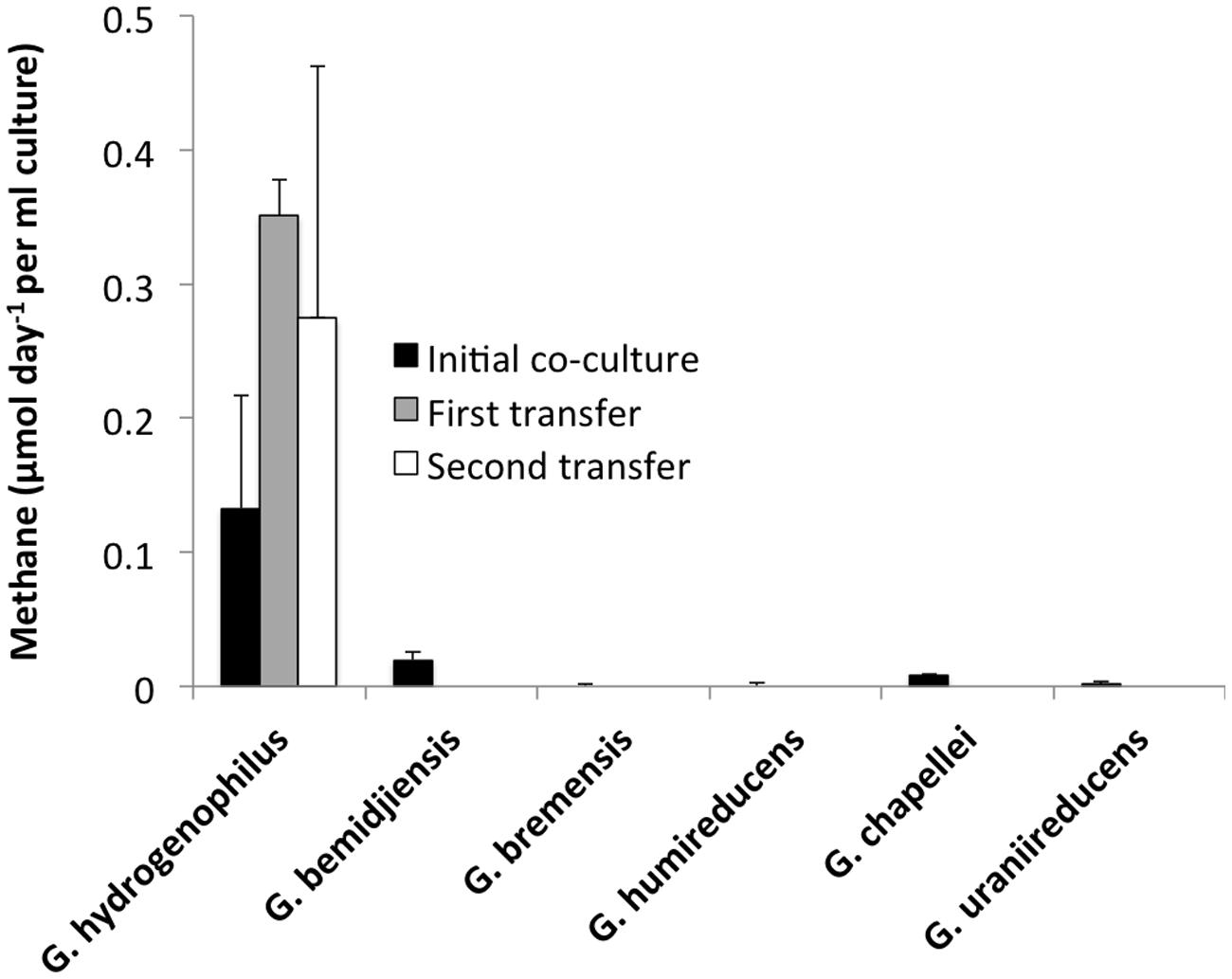
FIGURE 4. Rates of methane evolution from ethanol in co-cultures of M. barkeri with different Geobacter species during the initial co-cultivation (black bars) and the subsequent two transfers for the successful G. hydrogenophilus – M. barkeri co-culture. Results show the means and SD for triplicate co-cultures of each Geobacter species, incubated for a minimum of 100 days.
As previously observed with co-cultures established between G. metallireducens and M. barkeri (Rotaru et al., 2014b), there was a long lag prior to detectable methane production in G. hydrogenophilus–M. barkeri co-cultures (Figure 3A). However, over time the co-culture adapted to steadily produce methane and could be successively transferred with sustained methane production (Figure 3B). The rates of methane production (0.9 ± 0.6 μmol per day) by G. hydrogenophilus co-cultured with M. barkeri were lower, but comparable to rates previously observed (Rotaru et al., 2014b) in co-cultures of G. metallireducens and M. barkeri (2.7 ± 0.3 μmol per day).
Several lines of evidence suggested that G. hydrogenophilus and M. barkeri exchanged electrons via DIET. For example, like G. metallireducens (Rotaru et al., 2014b), G. hydrogenophilus appeared to be incapable of exchanging electrons via H2 or formate because it did not form a successful co-culture with the strict H2/formate-utilizing methanogen Methanospirillum hungatei, even after 150 days of incubation (See Supplementary Materials). Furthermore, GAC greatly accelerated electron transfer between G. hydrogenophilus and M. barkeri (Figure 5A) compared to co-cultures initiated at the same time without GAC (Figure 5). The high conductivity of GAC promotes DIET (Liu et al., 2012; Rotaru et al., 2014b), but similar to other conductive materials, GAC is not expected to enhance interspecies H2 transfer (Chen et al., 2014a).
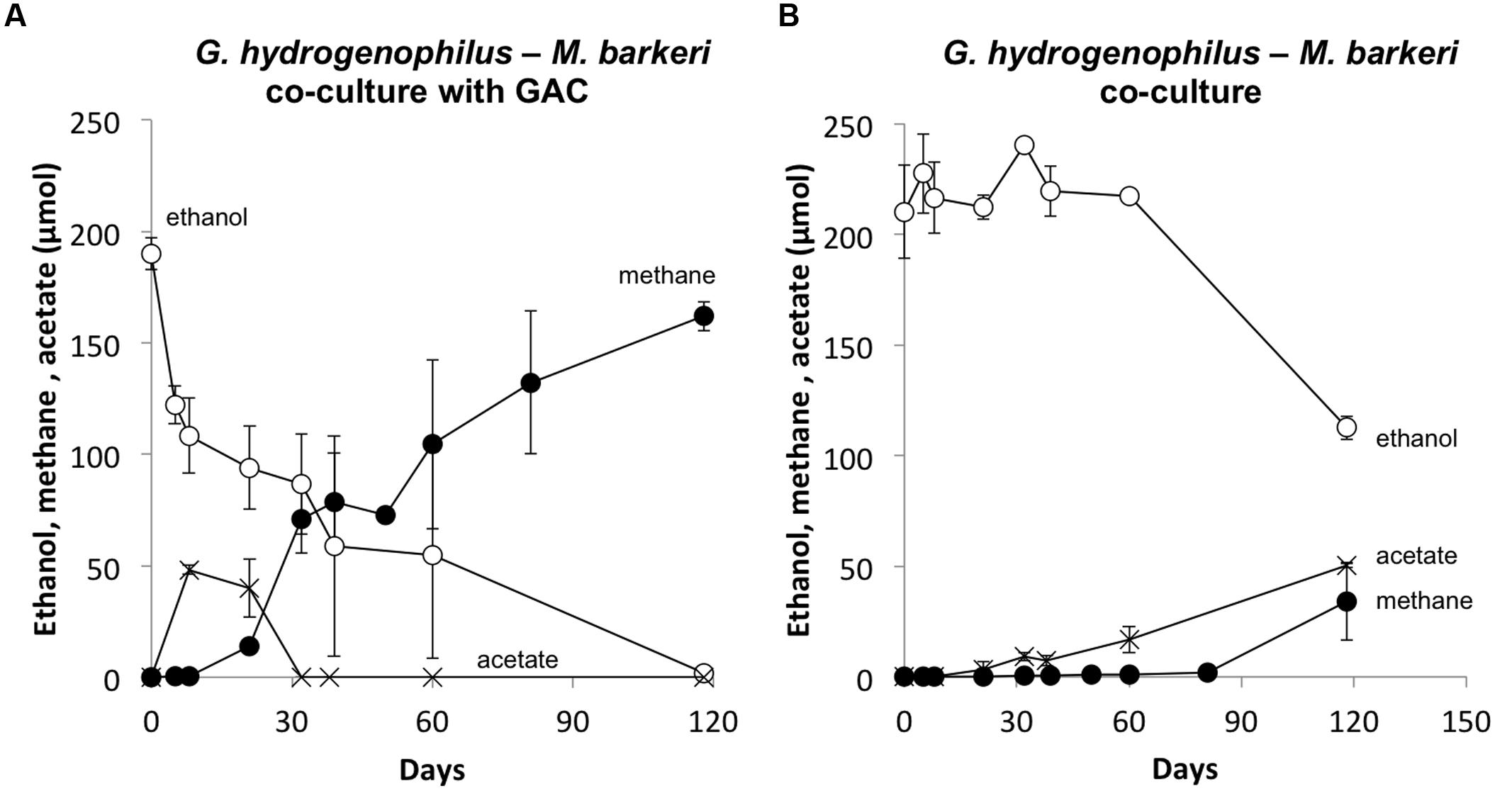
FIGURE 5. Methane and acetate formation from ethanol in co-cultures of G. hydrogenophilus and M. barkeri with (A) or without (B) granular activated carbon. Results are the mean and SD for triplicate incubations.
The availability of systems for genetic manipulation of G. sulfurreducens and G. metallireducens made if possible to further confirm electron transfer via DIET with deletions of genes for key extracellular electron transfer components (Summers et al., 2010; Rotaru et al., 2012, 2014a,b; Shrestha et al., 2013b). However, a strategy for genetic manipulation of G. hydrogenophilus has yet to be developed.
Implications
The results demonstrate that Geobacter species differ substantially in their capacities for current production and Fe(III) oxide reduction, as well as their ability to form syntrophic associations via DIET. Among the species tested, the effectiveness for Fe(III) reduction was a poor predictor of their ability for current production. In contrast, the three species of Geobacter that produce the highest current densities are the only three Geobacter species among those tested to date that can participate in DIET.
The correspondence between the capacity for syntrophic growth and the ability to produce high current densities suggests that there are commonalities in these two types of extracellular electron exchange and that the prior evolution of some Geobacter species for syntrophic growth via DIET conferred characteristics that permit these species to effectively utilize electrodes as electron acceptors. Although electrically conductive pili are one component that is essential for high current densities and DIET (Malvankar and Lovley, 2014), it is likely that other extracellular electron transfer components, as well as features that favor cell aggregation/biofilm formation, are also important. Therefore, further elucidation of the mechanisms for DIET may also provide insights into how electrons are transferred through conductive electrode biofilms and vice versa.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to credit Beatrice Markovaite for lab assistance. We are thankful to Joy Ward for lab management. The Office of Naval Research, grant no. N000141310549, funded this work. The first author was supported during the writing of this manuscript by the Danish Research Council award no. DFF-132500025.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2015.00744
References
Anderson, R. T., and Lovley, D. R. (1999). Naphthalene and benzene degradation under Fe (III)-reducing conditions in petroleum-contaminated aquifers. Bioremediat. J. 3, 121–135. doi: 10.1080/10889869991219271
Anderson, R. T., Vrionis, H. A., Ortiz-Bernad, I., Resch, C. T., Long, P. E., Dayvault, R., et al. (2003). Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69, 5884–5891. doi: 10.1128/AEM.69.10.5884-5891.2003
Balch, W. E., Fox, G., Magrum, L., Woese, C., and Wolfe, R. (1979). Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43, 260–296.
Butler, J., Young, N., and Lovley, D. (2010). Evolution of electron transfer out of the cell: comparative genomics of six Geobacter genomes. BMC Genomics 11:40. doi: 10.1186/1471-2164-11-40
Call, D. F., and Logan, B. E. (2011). A method for high throughput bioelectrochemical research based on small scale microbial electrolysis cells. Biosens. Bioelectron. 26, 4526–4531. doi: 10.1016/j.bios.2011.05.014
Chen, S., Rotaru, A.-E., Liu, F., Philips, J., Woodard, T., Nevin, K. P., et al. (2014a). Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour. Technol. 173, 83–86. doi: 10.1016/j.biortech.2014.09.009
Chen, S., Rotaru, A.-E., Shrestha, P. M., Malvankar, N. S., Liu, F., Fan, W., et al. (2014b). Promoting interspecies electron transfer with biochar. Sci. Rep. 4, 5019. doi: 10.1038/srep05019
Childers, S. E., Ciufo, S., and Lovley, D. R. (2002). Geobacter metallireducens accesses insoluble Fe (III) oxide by chemotaxis. Nature 416, 767–769. doi: 10.1038/416767a
Coates, J. D., Bhupathiraju, V. K., Achenbach, L. A., Mclnerney, M., and Lovley, D. R. (2001). Geobacter hydrogenophilus, Geobacter chapellei and Geobacter grbiciae, three new, strictly anaerobic, dissimilatory Fe (III)-reducers. Int. J. Syst. Evol. Microbiol. 51, 581–588.
Cruz Viggi, C., Rossetti, S., Fazi, S., Paiano, P., Majone, M., and Aulenta, F. (2014). Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 48, 7536–7543. doi: 10.1021/es5016789
Franks, A. E., Glaven, R. H., and Lovley, D. R. (2012). Real-time spatial gene expression analysis within current-producing biofilms. ChemSusChem 5, 1092–1098. doi: 10.1002/cssc.201100714
Holmes, D. E., Giloteaux, L., Chaurasia, A. K., Williams, K. H., Luef, B., Wilkins, M. J., et al. (2015). Evidence of Geobacter-associated phage in a uranium-contaminated aquifer. ISME J. 9, 333–346. doi: 10.1038/ismej.2014.128
Holmes, D. E., O’neil, R. A., Vrionis, H. A., N’guessan, L. A., Ortiz-Bernad, I., Larrahondo, M. J., et al. (2007). Subsurface clade of Geobacteraceae that predominates in a diversity of Fe(III)-reducing subsurface environments. ISME J. 1, 663–677. doi: 10.1038/ismej.2007.85
Hori, T., Noll, M., Igarashi, Y., Friedrich, M. W., and Conrad, R. (2007). Identification of acetate-assimilating microorganisms under methanogenic conditions in anoxic rice field soil by comparative stable isotope probing of RNA. Appl. Environ. Microbiol. 73, 101–109. doi: 10.1128/AEM.01676-06
Kato, S., Hashimoto, K., and Watanabe, K. (2012). Methanogenesis facilitated by electric syntrophy via (semi) conductive iron-oxide minerals. Environ. Microbiol. 14, 1646–1654. doi: 10.1111/j.1462-2920.2011.02611.x
Kumar, R., Singh, L., Wahid, Z. A., and Din, M. F. M. (2015). Exoelectrogens in microbial fuel cells toward bioelectricity generation: a review. Int. J. Energy Res. 39, 1048–1067. doi: 10.1002/er.3305
Leang, C., Malvankar, N. S., Franks, A. E., Nevin, K. P., and Lovley, D. R. (2013). Engineering Geobacter sulfurreducens to produce a highly cohesive conductive matrix with enhanced capacity for current production. Energy Environ. Sci. 6, 1901–1908. doi: 10.1039/c3ee40441b
Li, H., Chang, J., Liu, P., Fu, L., Ding, D., and Lu, Y. (2014). Direct interspecies electron transfer accelerates syntrophic oxidation of butyrate in paddy soil enrichments. Environ. Microbiol. 17, 1533–1547. doi: 10.1111/1462-2920.12576
Liu, F., Rotaru, A.-E., Shrestha, P. M., Nevin, K., and Lovley, D. (2012). Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 8982–8989. doi: 10.1039/c2ee22459c
Liu, X., Tremblay, P.-L., Malvankar, N. S., Nevin, K. P., Lovley, D. R., and Vargas, M. (2014). A Geobacter sulfurreducens strain expressing Pseudomonas aeruginosa type IV pili localizes OmcS on pili but is deficient in Fe (III) oxide reduction and current production. Appl. Environ. Microbiol. 80, 1219–1224. doi: 10.1128/AEM.02938-13
Lovley, D. R., and Malvankar, N. S. (2015). Seeing is believing: novel imaging techniques help clarify microbial nanowire structure and function. Environ. Microbiol. doi: 10.1111/1462-2920.12708 [Epub ahead of print].
Lovley, D. R., and Phillips, E. J. (1987). Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 53, 1536–1540.
Lovley, D. R., and Phillips, E. J. (1988). Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54, 1472–1480.
Lovley, D., Ueki, T., Zhang, T., Malvankar, N., Shrestha, P., Flanagan, K., et al. (2011). Geobacter: the microbe electric’s physiology, ecology, and practical applications. Adv. Microb. Physiol. 59, 1–100. doi: 10.1016/B978-0-12-387661-4.00004-5
Mahadevan, R., Palsson, B. Ø., and Lovley, D. R. (2011). In situ to in silico and back: elucidating the physiology and ecology of Geobacter spp. using genome-scale modelling. Nat. Rev. Microbiol. 9, 39–50. doi: 10.1038/nrmicro2456
Malvankar, N. S., and Lovley, D. R. (2014). Microbial nanowires for bioenergy applications. Curr. Opin. Biotechnol. 27, 88–95. doi: 10.1016/j.copbio.2013.12.003
Malvankar, N. S., Vargas, M., Nevin, K. P., Franks, A. E., Leang, C., Kim, B.-C., et al. (2011). Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 6, 573–579. doi: 10.1038/nnano.2011.119
Morita, M., Malvankar, N. S., Franks, A. E., Summers, Z. M., Giloteaux, L., Rotaru, A. E., et al. (2011). Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. MBio 2, e00159–e00211. doi: 10.1128/mBio.00159-11
Nevin, K. P., Holmes, D. E., Woodard, T. L., Hinlein, E. S., Ostendorf, D. W., and Lovley, D. R. (2005). Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., two novel Fe (III)-reducing subsurface isolates. Int. J. Syst. Evol. Microbiol. 55, 1667–1674. doi: 10.1099/ijs.0.63417-0
Nevin, K. P., Kim, B.-C., Glaven, R. H., Johnson, J. P., Woodard, T. L., Methé, B. A., et al. (2009). Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS ONE 4:e5628. doi: 10.1371/journal.pone.0005628
Nevin, K. P., Richter, H., Covalla, S., Johnson, J., Woodard, T., Orloff, A., et al. (2008). Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ. Microbiol. 10, 2505–2514. doi: 10.1111/j.1462-2920.2008.01675.x
Reguera, G., Mccarthy, K. D., Mehta, T., Nicoll, J. S., Tuominen, M. T., and Lovley, D. R. (2005). Extracellular electron transfer via microbial nanowires. Nature 435, 1098–1101. doi: 10.1038/nature03661
Reguera, G., Nevin, K. P., Nicoll, J. S., Covalla, S. F., Woodard, T. L., and Lovley, D. R. (2006). Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 72, 7345–7348. doi: 10.1128/AEM.01444-06
Richter, H., Mccarthy, K., Nevin, K. P., Johnson, J. P., Rotello, V. M., and Lovley, D. R. (2008). Electricity generation by Geobacter sulfurreducens attached to gold electrodes. Langmuir 24, 4376–4379. doi: 10.1021/la703469y
Rotaru, A.-E., Shrestha, P. M., Liu, F., Markovaite, B., Chen, S., Nevin, K. P., et al. (2014a). Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 80, 4599–4605. doi: 10.1128/AEM.00895-14
Rotaru, A.-E., Shrestha, P. M., Liu, F., Shrestha, M., Shrestha, D., Embree, M., et al. (2014b). A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 7, 408–415. doi: 10.1039/C3EE42189A
Rotaru, A.-E., Shrestha, P. M., Liu, F., Ueki, T., Nevin, K., Summers, Z. M., et al. (2012). Interspecies electron transfer via hydrogen and formate rather than direct electrical connections in cocultures of Pelobacter carbinolicus and Geobacter sulfurreducens. Appl. Environ. Microbiol. 78, 7645–7651. doi: 10.1128/AEM.01946-12
Shelobolina, E. S., Vrionis, H. A., Findlay, R. H., and Lovley, D. R. (2008). Geobacter uraniireducens sp. nov., isolated from subsurface sediment undergoing uranium bioremediation. Int. J. Syst. Evol. Microbiol. 58, 1075–1078. doi: 10.1099/ijs.0.65377-0
Shrestha, P., Malvankar, N., Werner, J., Franks, A., Rotaru, A., Shrestha, M., et al. (2014). Correlation between microbial community and granule conductivity in anaerobic bioreactors for brewery wastewater treatment. Bioresour. Technol. 174, 306–310. doi: 10.1016/j.biortech.2014.10.004
Shrestha, P. M., and Rotaru, A.-E. (2014). Plugging in or going wireless: strategies for interspecies electron transfer. Front. Microbiol. 5:237. doi: 10.3389/fmicb.2014.00237
Shrestha, P. M., Rotaru, A., Aklujkar, M., Liu, F., Shrestha, M., Summers, Z., et al. (2013a). Syntrophic growth with direct interspecies electron transfer as the primary mechanism for energy exchange. Environ. Microbiol. Rep. 5, 904–910. doi: 10.1111/1758-2229.12093
Shrestha, P. M., Rotaru, A.-E., Summers, Z. M., Shrestha, M., Liu, F., and Lovley, D. (2013b). Transcriptomic and genetic analysis of direct Interspecies electron transfer. Appl. Environ. Microbiol. 79, 2397–2404. doi: 10.1128/AEM.03837-12
Straub, K. L., and Buchholz-Cleven, B. (2001). Geobacter bremensis sp. nov. and Geobacter pelophilus sp. nov., two dissimilatory ferric-iron-reducing bacteria. Int. J. Syst. Evol. Microbiol. 51, 1805–1808. doi: 10.1099/00207713-51-5-1805
Summers, Z. M., Fogarty, H. E., Leang, C., Franks, A. E., Malvankar, N. S., and Lovley, D. R. (2010). Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330, 1413–1415. doi: 10.1126/science.1196526
Tremblay, P. L., Aklujkar, M., Leang, C., Nevin, K. P., and Lovley, D. (2012). A genetic system for Geobacter metallireducens: role of the flagellin and pilin in the reduction of Fe (III) oxide. Environ. Microbiol. Rep. 4, 82–88. doi: 10.1111/j.1758-2229.2011.00305.x
Ueki, T., Leang, C., Inoue, K., and Lovley, D. R. (2012). Identification of multicomponent histidine-aspartate phosphorelay system controlling flagellar and motility gene expression in Geobacter species. J. Biol. Chem. 287, 10958–10966. doi: 10.1074/jbc.M112.345041
Vargas, M., Malvankar, N. S., Tremblay, P.-L., Leang, C., Smith, J. A., Patel, P., et al. (2013). Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens. MBio 4, e00105–e00113. doi: 10.1128/mBio.00210-13
Xu, J., Zhuang, L., Yang, G., Yuan, Y., and Zhou, S. (2013). Extracellular quinones affecting methane production and methanogenic community in paddy soil. Microb. Ecol. 66, 950–960. doi: 10.1007/s00248-013-0271-7
Yates, M. D., Kiely, P. D., Call, D. F., Rismani-Yazdi, H., Bibby, K., Peccia, J., et al. (2012). Convergent development of anodic bacterial communities in microbial fuel cells. ISME J. 6, 2002–2013. doi: 10.1038/ismej.2012.42
Keywords: Geobacter, Methanosarcina, syntrophy, direct interspecies electron transfer, electrogen
Citation: Rotaru A-E, Woodard TL, Nevin KP and Lovley DR (2015) Link between capacity for current production and syntrophic growth in Geobacter species. Front. Microbiol. 6:744. doi: 10.3389/fmicb.2015.00744
Received: 28 February 2015; Accepted: 06 July 2015;
Published: 21 July 2015.
Edited by:
Martin G. Klotz, The City University of New York, USAReviewed by:
Daniel Hassett, University of Cincinnati, USATom Clarke, University of East Anglia, UK
Copyright © 2015 Rotaru, Woodard, Nevin and Lovley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amelia-Elena Rotaru, Nordic Center for Earth Evolution, Department of Biology, University of Southern Denmark, Campussvej 55, Odense 5230, Denmark, arotaru@biology.sdu.dk
 Amelia-Elena Rotaru
Amelia-Elena Rotaru Trevor L. Woodard
Trevor L. Woodard Kelly P. Nevin
Kelly P. Nevin Derek R. Lovley
Derek R. Lovley