- 1Section of Microbiology and Medical Genetics, Department of Medical Sciences, University of Ferrara, Ferrara, Italy
- 2Neuroradiology Unit, Department of Neurosciences and Rehabilitation, Azienda Ospedaliera-Universitaria Arcispedale S. Anna, Ferrara, Italy
Human leukocyte antigen (HLA)-G molecule, a non-classical HLA-Ib molecule, is less polymorphic when compared to classical HLA class I molecules. Human leukocyte antigen-G (HLA-G) was first detected on cytotrophoblast cells at the feto-maternal interface but its expression is prevalent during viral infections and several autoimmune diseases. HLA-G gene is characterized by polymorphisms at the 3′ un-translated region and 5′ upstream regulatory region that regulate its expression and are associated with autoimmune diseases and viral infection susceptibility, creating an unbalanced and pathologic environment. This review focuses on the role of HLA-G genetic polymorphisms, mRNA, and protein expression in autoimmune conditions and viral infections.
Introduction
Human Leukocyte Antigen-G (HLA-G) is a functional molecule belonging to class Ib human leukocyte antigens (HLA) characterized by a non-covalent link between β2-microglobulin (β2m) and glycoprotein heavy chain. The gene is located within Major Histocompatibility Complex (MHC) locus on chromosome 6 (1, 2). HLA-G products show some peculiar features for which they are considered as non-classical HLA-I antigens: (1) the limitation of their allelic polymorphism (3); (2) the expression of seven isoforms represented by four membrane-bound (G1, G2, G3, and G4) and three soluble (G5, G6, and G7) proteins (4); and (3) the restriction of their tissue distribution (5). Polymorphisms at the 5′ upstream regulatory region and at the 3′ UTR of the HLA-G gene play an important role in the regulation of HLA-G production (6). Mainly, two polymorphisms at the 3′ UTR: a deletion/insertion (DEL/INS) of 14 base pairs (14bp) polymorphism (rs371194629) and a C > G single-nucleotide polymorphism (SNP) at the +3142bp position (rs1063320) (7) (Figure 1) are able to affect mRNA stability in vivo and protein production and implicated in pathological conditions: 14bpINS allele is associated with mRNA instability (8, 9); +3142G allele creates a binding site for three microRNAs (miRNAs) (miR-148a, miR-148b, and miR-152) reducing soluble protein production (10). These observations suggest that 14bpINS/INS and +3142G/G genotypes are associated with a lower HLA-G production than 14bpDEL/INS and DEL/DEL, +3142C/G, and C/C genotypes (8, 10).
Membrane-bound HLA-G1 and soluble HLA-G5 (HLA-G5) represent the mainly expressed and investigated HLA-G isoforms (1) and are currently supposed to be the most important and functional isoforms (11). However, while HLA-G5 molecules are actively secreted as soluble isoforms, HLA-G1 proteins could be released by proteolytic shedding from cell surface (sHLA-G1) via matrix metalloproteinase-2 (MMP-2) (12–16). HLA-G can exist as β2m-associated and -free monomers (17, 18) and as disulfide-linked dimers or multimers (17, 19, 20). HLA-G disulfide-linked dimers are linked by disulfide bonds between two cysteine residues at position 42 of the HLA-G alpha-1 domain (19–21) and present higher affinity for ILT-2 and ILT-4 receptors compared to monomers (22, 23). Placental trophoblast cells (24), thymus (25), cornea (26), nail matrix (27), pancreas (28), erythroid, and endothelial precursors (29) present a physiological expression of HLA-G molecules. However, HLA-G can be ectopically expressed also on monocytes (30), in transplantation, tumors, viral infections, and autoimmune diseases (1, 2). HLA-G antigens are currently considered as immune-modulatory molecules due to their role in preserving immune tolerance at the feto-maternal interface (31), promoting graft tolerance (32), reducing inflammatory and immune responses (33), favoring tumors (34), and virus infection via immune escape (35). Both membrane-bound and soluble HLA-G antigens exert their immune-suppressive properties: (a) inhibiting the activity and inducing apoptosis of cytotoxic CD8+ T cells and NK cells (36–38); (b) inhibiting the proliferation of CD4+ T cells that are shifted to an immune-suppressive profile (39, 40); (c) inhibiting antigen-presenting cells and B cell differentiation (41, 42); (d) inducing a Th2 polarization (43); and (e) inducing regulatory T cells (44) and Interleukin (IL)-10 secreting dendritic cells (DC10) (45) (Figure 2). The interactions between HLA-G proteins and their specific inhibitory receptors ILT-2 (LILRB1/CD85j), ILT-4 (LILRB2/CD85d), and KIR2DL4 (CD158d) expressed by immune cells (46) account for the effects of these molecules on immune cells.
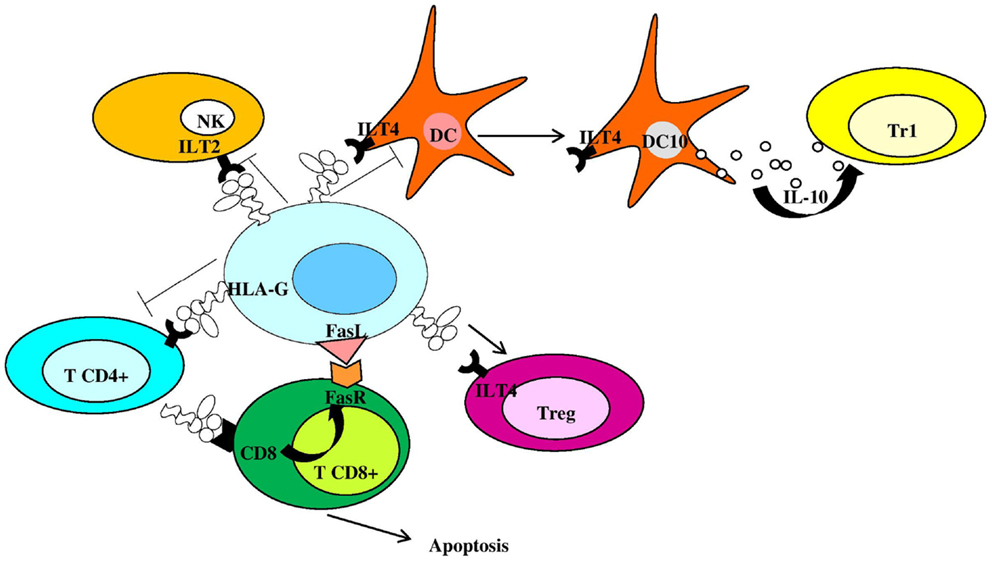
Figure 2. Human leukocyte antigen-G is an anti-inflammatory molecule inhibiting and controlling immune cell activation. NK, natural killer cells; Tr1, type 1 regulatory T cells; DC, dendritic cell; Treg, regulatory T cell; FasR, Fas receptor; DC10, IL-10-differentiated dendritic cells.
Moreover, HLA-G expression is up-regulated by the secretion of anti-inflammatory cytokines such as IL-10 which, in its turn, is enhanced by HLA-G (30). For these reasons, the implication of HLA-G molecules in inflammatory, immune-mediated, and infective conditions has been investigated (47, 48). The knowledge of the interactions between HLA-G molecules and immune mechanisms and their implication in pathological conditions may assist in improving our knowledge on the mechanisms at the basis of several autoimmune diseases and viral infections.
HLA-G and Gastrointestinal Diseases
Celiac disease is a gluten sensitivity, which induces an inflammation that damages the villi in the small intestine of genetically predisposed subjects. Both genetic and environmental factors contribute to the development of celiac disease (CD). Torres and coauthors (49) have shown the presence of HLA-G in biopsies from celiac patients and have observed higher sHLA-G amounts in comparison with control subjects. The evaluation of the 14bp INS/DEL polymorphism in a group of 522 celiac patients (50), subdivided accordingly with the presence of HLA-DQ2 molecule, encoded by DQA1*05/DQB1*02 genes, has demonstrated an increased frequency of the 14bp INS/INS genotype in comparison with controls. These data suggest that the 14bp INS allele may increase the risk of gut inflammation, most likely leading to chronicity. Ulcerative colitis (UC) and Crohn’s disease are characterized by a different sHLA-G expression pattern (51) by peripheral blood mononuclear cells. Non-activated peripheral blood mononuclear cells from Crohn’s disease patients secrete spontaneously sHLA-G while those from UC patients and healthy donors do not. Furthermore, after stimulation with LPS, both cells from Crohn’s disease and healthy subjects show sHLA-G production, while this does not happen in UC patients. The different HLA-G expression profiles in UC and Crohn’s disease patients sustain the different aethiopathogenesis at the origin of these two diseases. In particular, the responses to therapies in UC and Crohn’s disease correspond to different sHLA-G secretion levels (52). The immunosuppressant therapy normalizes the production of HLA-G molecules in Crohn’s disease while it starts the release of HLA-G in UC patients. These data confirm the diversity in the behavior of these two pathologies and propose the analysis of sHLA-G levels with the final goal of distinguishing between UC and Crohn’s disease patients and to monitor therapy.
HLA-G and Rheumatologic Diseases
Rheumatic diseases are inflammatory and autoimmune diseases, which are the second most common cause of disability after musculoskeletal injuries. Rheumatoid arthritis (RA) is an autoimmune disease caused by the immune system attacking synovial cells. A combination of genetic and environmental factors may increase the risk of RA. Gene expression profiles (GEPs) in bone marrow-derived RA mononuclear cells (53) have shown 1,910 down-regulated and 764 up-regulated gene, which include the HLA-G gene. Several studies have evaluated the role of HLA-G polymorphisms in RA susceptibility without reaching a final common result. The evaluation on 256 RA patients and 356 healthy controls genotyped for the HLA-G 14bp INS/DEL polymorphism has reported no differences in allelic and genotypic frequencies and no correlation with disease characteristics (54). The analysis of two SNPs (rs1736936, −1305G/A and rs2735022, −689A/G) in HLA-G promoter in the Korean population has not presented any connection to the development of RA (55). The evaluation in a Brazilian cohort documented the implication of 3′ UTR polymorphisms in RA follow-up (56). The authors have observed a significant association of the −762C > T, −716T > G, −689A > G, −666G > T, −633G > A, −486A > C, and −201G > A (rs1632946; rs2249863; rs2735022; rs35674592; rs1632944; rs1736933; and rs1233333) SNPs with the disease. The analysis of 106 patients with juvenile idiopathic arthritis (JIA) has shown an association between JIA female susceptibility and the 14 bp DEL allele. These different associations support the presence of different pathogenic elements between RA and JIA (54). RA (57) and JIA patients present lower serum sHLA-G concentration than in controls (58), with a possible contribution to the chronicity of the inflammation. On the contrary, JIA synovial fluids showed higher sHLA-G levels than controls (SF) (56). Since we have observed that HLA-G molecules are enhanced in synovial fibroblasts from inflamed joints (59) and that high sHLA-G levels correlate with disease activity (57), we may suggest an impaired control of immune reaction at joint, which characterizes JIA disease. The HLA-G 14bp INS/DEL polymorphism has also been evaluated as a marker for RA therapy. Methotrexate (MTX), a disease-modifying anti-rheumatic drug (DMARD), induces an increased production of IL-10 in RA patients with a better therapeutic response (60) and is able to enhance HLA-G secretion by peripheral blood mononuclear cells (61). Interestingly, the 14bp DEL/DEL genotype is increased in RA patients with a good response to MTX therapy (62), with a possible implication in the control of immune activation. It must be underlined, however, that contrasting results have been obtained (63, 64), possibly due to a different dosage of MTX, a different cut-off value for RA therapy response assessment. Scleroderma (SSc) is an autoimmune rheumatic disease of the connective tissue (65). Only SSc patients with a longer survival, lower frequency of vascular cutaneous ulcers, telangiectasias, and inflammatory polyarthralgia present HLA-G molecule expression in skin biopsies (66) suggesting an implication of this molecule on the control of immune response at the skin level.
Systemic lupus erythematosus is a systemic autoimmune disease of the connective tissue that can affect any part of the body. The immune response is mainly characterized by Th2-cell predominance. Rosado and coauthors (67) and Chen and coauthors (68) have shown higher sHLA-G and IL-10 levels in systemic lupus erythematosus (SLE) patients in comparison with healthy controls, while Rizzo and coauthors (69) have observed lower sHLA-G concentrations in SLE patients (70). Interesting, the analysis of monocytes and mature CD83 positive dendritic cells from SLE patients has evidenced a diminished expression of HLA-G in comparison with healthy controls (71), a lower HLA-G expression in response to IL-10 and a lower HLA-G trogocytosis from autologous monocytes compared with controls. Using the SNPs mapping approach, HLA-G gene is recognized as a novel independent locus for SLE (72). In particular, HLA-G 14bp INS/DEL polymorphism and HLA-G +3142C > G SNP have been analyzed in a SLE population. SLE patients showed a higher frequency of 14bp INS allele and 14bp INS/INS genotype (69) and the heterozygote group showed lower systemic lupus erythematosus disease activity index (SLEDAI) indexes than homozygous groups (73). On the contrary, the evaluation of HLA-G 14bp INS/DEL polymorphism in a SLE Brazilian population did not present an association (74), while the +3142G allele and the +3142 GG genotype frequencies were increased among SLE patients as compared with controls (75, 76). These data sustain a possible role of HLA-G expression in modifying SLE condition. Behçet (BD) and Kawasaki diseases are autoimmune vasculitis. The HLA-G*01:01:01 allele is associated with a reduced risk of BD while HLA-G*01:01:02 and G*01:05N alleles are associated with an increased risk of BD (77, 78). Non-synonymous SNP (+755A/C) of the HLA-G gene (rs12722477, G*01:04) is significantly associated with Kawasaki disease (79). These data suggest an influence of HLA-G polymorphisms in determining disease risk, possibly affecting HLA-G production and consequently inflammation status.
HLA-G and Cutaneous Diseases
The skin is characterized by a “skin immune system (SIS),” where immune cells and humoral components support cutaneous inflammation. The deregulation of skin defense mechanisms is evident in a large variety of inflammatory disorders of the skin, such as psoriasis, atopic dermatitis, pemfigo, vitiligo, and systemic sclerosis (80). HLA-G protein is not expressed in the skin from healthy controls (81, 82). Ectopic HLA-G expression has been described in skin pathologies (83–86).
Psoriasis is a chronic inflammatory skin disease with an autoimmune component. Both membrane-bound and soluble HLA-G proteins have been detected in psoriatic skin lesions with the main compound characterized by macrophage lining at the dermo-epidermal junctions (82). The up-regulation of HLA-G molecules by macrophages could represent an attempt to control auto-reactive T cells, induced by activated keratinocytes-derived cytokines/chemokines. HLA-G may prevent keratinocyte destruction by modulating the activity of cytotoxic lymphocytes and promoting the development of Treg cells (87). Interestingly, significantly lower plasma sHLA-G levels have been found in psoriatic patients compared with controls (88), suggesting a difference in systemic HLA-G expression that could be associated with the IL-10 deficiency typical of psoriasis. Psoriasis management can be divided into three main types: topical drugs, light therapy, and systemic medications. Evaluation of therapeutic effects on sHLA-G expression has shown an increase in plasmatic levels of systemic treated patients (efalizumab, cyclosporin A, and acitretin) (88) and a significant association between HLA-G 14bp DEL allele and 14bp DEL/DEL genotype with acitretin clinical outcome (89). We can suppose a possible direct effect of HLA-G in antagonizing systemic T helper 1 activation and with a potential role as a marker of response to acitretin in psoriatic patients.
Pemphigus vulgaris is a blistering disease caused by autoantibodies to desmoglein skin adhesion proteins. Skin tissue sections from pemphigus vulgaris (PV) patients express detectable HLA-G molecules at both transcriptional and translational levels, while control sections present only HLA-G transcription (90). Moreover, the HLA-G 14bp DEL allele has been observed with higher frequency in PV patients in comparison with controls in a Jewish population (91). These data suggest that HLA-G expression could be a detrimental factor for the development of PV.
HLA-G and Diabetes
Type 1 and type 2 diabetes present immunologic defects that enhance insulin resistance as a result of genetics sedentary lifestyle, obesity, and other conditions, such as chronic inflammation or infection. It has been shown that higher levels of sHLA-G are frequent in subjects with an impaired glucose metabolism (92). These data suggest a possible implication of HLA-G antigens in the diabetic condition. In fact, SNPs rs4122198, rs2394186, rs1619379, and rs1611133 near the HLA-G gene have been associated with type 1 diabetes (93); dendritic cells from type 1 diabetic patients produce lower HLA-G molecules in response to IFN-beta (94) in comparison with control subjects and the HLA-G 14bp INS-INS genotype might contribute to the development of high blood pressure in type 2 diabetes (95).
Interestingly, HLA-G has been found in some secretory granules and on the cell surface of primary islet cells induced to secrete insulin (28). On the basis of these data, it could be hypothesized that an impaired HLA-G expression at pancreatic islets could sustain T cell activation and onset of diabetes.
HLA-G in Multiple Sclerosis
Multiple sclerosis is the prototypic autoimmune disease of the central nervous system (CNS) characterized by chronic inflammatory demyelination and neurodegeneration of unidentified origin (96). Multiple sclerosis (MS) typically occurs in young adults and manifests in women twice as frequently as in men with neurological symptoms and signs, called relapses, which are usually disseminated in space and time (97). About the 80% of MS patients present a disease onset with a relapsing–remitting (RR) form followed by a secondary progressive (SP) course that arises after years, whereas MS starts with a primary progressive (PP) form in approximately the 20% of subjects (98). However, the recent proposed criteria (99) suggest that the coexistence of multi-focal lesions in the periventricular white matter on T2-weighted Magnetic Resonance Imaging (MRI) scans with or without Gadolinium (Gd) enhancement on T1-weighted MRI scans are needed for the diagnosis of MS. Based on epidemiological studies, exposure to an environmental factor, e.g., an infectious agent, in genetically predisposed individuals is currently thought to be crucial for MS pathogenesis (100) in which the traffic into the CNS of activated auto-reactive CD4+ T helper 1 (Th1) cells plays a central role (96, 101, 102). The initiation of brain inflammation is due to the activation of microglia by infiltrating CD4+ T cells leading to the generation of Th1-mediated immune responses (IL-12/IFN-γ and IL-23/IL-17), while the resolution of neuroinflammation is triggered by astrocytes, which promote anti-inflammatory Th2-polarized responses (IL-10 and TGF-β) and the elimination of infiltrating immune cells through Fas/FasL-dependent apoptosis (96, 101) (Figure 3).
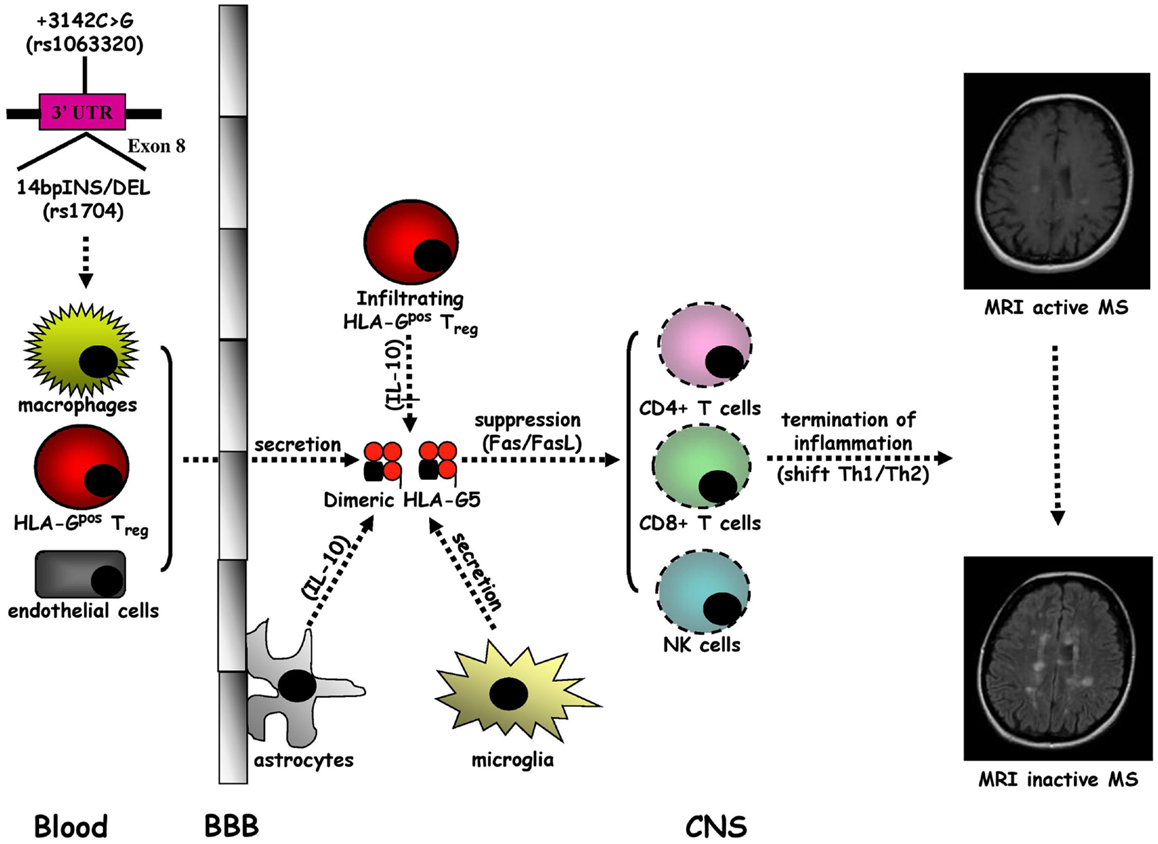
Figure 3. Intrathecal immune milieu in MS. The secretion of HLA-G5 in dimeric form by macrophages and HLA-Gpos Treg infiltrating the central nervous system (CNS) across the blood–brain barrier (BBB), endothelial cells, and microglia, sustained by a IL-10 release by astrocytes, may promote the suppression of CD4+ Th1 cell activity and the apoptotic removal of CD8+ T cells and NK cells that favor the formation of an anti-inflammatory intrathecal microenvironment leading to the termination of MS inflammation.
A growing body of evidence indicates that sHLA-G antigens may have a tolerogenic role in MS (102, 103). Cerebrospinal fluid (CSF) detectable sHLA-G has been detected in RRMS patients with higher levels in comparison with other inflammatory neurological disorders (OIND), non-inflammatory neurological disorders (NIND), and controls (104). Furthermore, higher CSF sHLA-G levels have been detected in RRMS without MRI evidence compared to those with MRI active disease. Notably, a positive correlation between CSF concentrations of sHLA-G and IL-10 has been found in MS patients without MRI evidence of active disease. Therefore, CSF levels of sHLA-G may act, together with IL-10, as anti-inflammatory molecules to regulate MS disease activity. The association between elevated CSF sHLA-G levels and clinical and MRI appearance of MS stable disease is supported by the intrathecal synthesis of sHLA-G in MS clinically and MRI inactive patients (105). We have found higher CSF levels of HLA-G5 and not of sHLA-G1 isoforms compared with controls and in presence rather than in absence of MRI Gd enhancing lesions (106) and an as well as inverse correlation between CSF levels of sHLA-G and anti-apoptotic sFas molecules in MS patients without MRI disease activity (107). Collectively, these results suggest a strong correlation between high CSF levels of sHLA-G antigens and the resolution of MS autoimmunity probably related to the anti-inflammatory properties of these molecules. The impact of HLA-G in MS pathogenesis was recently confirmed by other studies, which demonstrated that: (a) Th1 and Th2 cytokine production and CD4+ T cell proliferation are suppressed by HLA-G from MS patient peripheral blood monocytes during the first month of treatment with IFN-β (108); (b) MS disease activity during pregnancy may be modulated by tolerogenic properties of sHLA-G since post-partum serum sHLA-G levels are higher in MS patients without clinical attacks (109); and (c) microglia, macrophages, and endothelial cells located within and around MS lesions present a strong immunohistochemical expression of HLA-G and its inhibitory receptors (ILT-2 and ILT-4), with an elevated protein HLA-G expression on cultured human microglial cells after activation with Th1 pro-inflammatory cytokines (110). Meanwhile, a novel subpopulation of naturally occurring CD4+ and CD8+ regulatory T cells of thymic origin expressing HLA-G (HLA-Gpos Treg), has been characterized in MS patients with a suppressive activity through the secretion of HLA-G5 and the shedding of sHLA-G1 (111–113). Overall, these data sustain anti-inflammatory properties of sHLA-G molecules, and in particular HLA-G-5 isoform, which could lead to the remission of MS autoimmunity. Although it has been demonstrated that SNP rs4959039, a SNP in the downstream un-translated region of HLA-G gene is independently associated with MS susceptibility (114), the possible link between HLA-G genetic polymorphisms and MS has not been intensively explored (102, 103). Conflicting results have been obtained. Although no association between HLA-G gene polymorphism and MS or severity of the disease has been initially found (115), 14bpINS and −725G (rs1233334) alleles have been shown to be related to MS (116). However, a recent study, evaluating the influence of 14bpDEL/INS and +3142C > G HLA-G polymorphisms on CSF and serum sHLA-G production, has documented a correlation between HLA-G genetic polymorphisms and sHLA-G concentrations in both CSF and serum (117). These findings indicate that CSF and serum sHLA-G levels in MS could be affected by two main HLA-G polymorphisms. Moreover, preliminary results from our laboratory have demonstrated that, MS patients present dimeric sHLA-G form more frequently than control, in particular in MRI inactive MS patients (unpublished data), suggesting that large amounts of biologically active dimeric sHLA-G form could be released in CSF of MS patients, possibly induced by pharmacological treatment (118). Nevertheless, in a recent study no association was found between serum sHLA-G levels, disability progression, disease MRI activity, and time to conversion from clinically isolated syndrome (CIS) to clinically definite MS (119). These findings suggest that the use of sHLA-G levels in CSF should be taken into consideration as a prognostic marker for monitoring disease conversion, activity, progression, and response to therapy.
HLA-G Impact in Viral Infections
Even if host immune system present several mechanisms to control viral infections, the viruses have developed several strategies to counteract host immune defenses (120). HLA-G seems to be implicated in viral immune-escape from Natural Killer cells (121).
Human immunodeficiency virus type 1 (HIV-1) up-regulates HLA-G molecules and down-regulates classical HLA-A and -B. Studies have focused on the expression of HLA-G in monocytes, which are relevant as reservoirs of HIV-1, and in lymphocytes, which are more susceptible to infection by HIV-1. Monocytes from HIV-1 seropositive patients express HLA-G (122) with a possible association with antiretroviral therapy (HAART), since patients undergoing HAART present higher levels of HLA-G expression on monocytes in comparison with untreated and healthy subjects (122, 123). T cells obtained from HIV-1 seropositive individuals have been found to express HLA-G at a higher proportion (124) and behave like HLA-G+ Treg. Furthermore, on the basis of HLA-G genetics, it would seem that the HLA-G 14bpINS and +3142G polymorphisms affect the susceptibility to HIV (125) but not mother–child transmission (126) in African population.
Human cytomegalovirus is a herpes virus that persists in the host (127) by means of several strategies to evade the immune system. HLA-G expression is evidenced during viral reactivation in macrophages and astrocytoma cells (35) and the levels of expression on monocytes an in serum is higher during active human cytomegalovirus (HCMV) infection (128). This up-regulation is proposed to be associated with virus-encoded homologs of humanIL-10 (cmvIL-10) (129), which prevents NK cell recognition of infected cells.
There is also evidence to support also a role of HLA-G molecules in susceptibility and outcome of human papilloma virus (HPV) infections. The alleles HLA-G 14bp INS, +1537C (rs12722477), G*01:01, G*01:04, and G*01:06 have been associated with both high-grade squamous intraepithelial lesions and cervical cancer, while HLA-G 14bp DEL and +3142C alleles have been identified as protective (130–135). These results are in agreement with the low levels of HLA-G5 expression in cervical cancer (136). On the other hand, two researches recognized HLA-G 14bp DEL allele and +3142C as associated with increased risk of cervical cancer (137, 138), in agreement with increased expression of HLA-G in cervical cancer tissues (139) and with the spontaneous de-methylation of HLA-G promoter that allows immune-evasion and the development of precancerous cervical lesions (140). HLA-G has been also implicated in nasal polyposis development in the presence of HPV infection (141). Nasal polyps with HPV11 infection have shown HLA-G expression on epithelial cells, while no HLA-G expression has been observed in HPV negative polyps.
Neurotropic viruses such as herpes simplex virus-1 (HSV-1) and Rabdovirus (RABV) (142) induce the expression and up-regulation of membrane and soluble HLA-G molecules in actively infected neurons with a consequent protection toward host NK cells.
Hepatitis C virus (HCV) and Hepatitis B virus (HBV) seems to induce HLA-G expression to control host immune response (125, 143–148).
On the basis of these results, HLA-G proteins are expressed by virally infected cells as a mechanism to evade host immune control, preventing T cell and NK cell activation. The main challenge would be to block HLA-G up-regulation by viral infection, in order to allow the recognition by immune cells.
Interaction of HLA-G Molecules with Other HLA-Ib Molecules
Other HLA-Ib molecules have been identified: HLA-E and HLA-F (149, 150) characterized by a low genetic diversity as well as by a particular expression pattern, structural organization and functional profile.
Similar to HLA-G, HLA-E forms a complex with β2-microglobulin. HLA-E is known to play an important role as immune-modulator during pregnancy and transplantation (151), inhibiting immune responses by its interaction with CD8+ T cell receptors (TCRs) (152) and with the CD94/NKG2A inhibitory receptors of NK cells (153). Meanwhile, this molecule may present non-self antigens activating immune response (154).
Similar to other HLA molecules, HLA-F can form a complex with beta2 microglobuli and three splicing variants have been described. While the presence of HLA-G and HLA-E has been recently correlated with physiological and pathological conditions, the clinic-pathological significance of HLA-F is limited. HLA-F is expressed by peripheral blood B cells upon activation (155) and is detected in embryonic tissues, including the extravillous trophoblasts invading maternal deciduas, and in spermatozoids (156, 157) and in the serum of patients affected by tumors (158).
Only few data are available on the interaction of HLA-G molecules with the other HLA-Ib antigens. In physiological conditions, HLA-G molecules interact with HLA-E and co-operate to inhibit NK cells, mainly at feto-maternal interface, via interaction with ILT-2 and CD94/NKG2A, respectively (159). In pathological condition, the interaction between these two molecules facilitates the escape of tumor cells from NK cell recognition (160). In MS, HLA-G and HLA-E molecules are expressed by resident CNS cells and interact with NK cell and cytotoxic lymphocytes (161). HLA-G, -E, and -F expression by trophoblasts correlates with the protection of the fetus from destruction by the maternal immune system, suggesting a co-operation for fetal tissue preservation.
Conclusion
This review aims to focus on the key role of HLA-G molecules in autoimmune diseases and viral infections. The data herein summarized suggest that HLA-G may have a crucial role in the creation of an impaired immune response that characterizes these pathological conditions.
In fact, it appears even more evident that HLA-G proteins are involved in the regulation of the immune system during autoimmunity, such as gastrointestinal, skin, rheumatic and neurological diseases and in the immune-escape mechanisms during viral infections.
Here, we have reviewed a series of experimental and epidemiological studies that support the direct influence of HLA-G proteins on the balance of immune settings. On this basis, understanding the function of HLA-G in these disorders could help in the identification of new approaches to control HLA-G production.
For example, it is interesting to note that inflammatory cutaneous diseases present a disproportional expression of HLA-G molecules with respect to controls and that this could generate autoimmunity. Thus it appears that down/over-expression of HLA-G may not only act as an immunosuppressive and beneficial molecule but may also sustain an unbalanced immune stimulation and autoimmunity. With reference to bowel diseases especially, it appears clear that the different HLA-G expression levels could help in the differential diagnosis and consequently in the choice of appropriate treatment.
Furthermore, several studies have evidenced the possible role of sHLA-G antigens as a tolerogenic molecules in MS since their intrathecal production is associated with disease remission. It is of extreme importance to evaluate the role of HLA-G antigens in MS pathogenesis, in particular if they are implicated in disease progression or if they represent an indirect manifestation of MS inflammation of CNS. Still to be clarified are the functional differences between HLA-G5 and sHLA-G1, and whether dimers and monomers exert a different function in MS inflammatory disease activity. As far as viral infections are concerned, HLA-G could be considered a target for anti-viral treatment, so increased knowledge in this field could contribute to identifying different therapeutic strategies.
Collectively, the results emerging from the literature confirm the importance of the HLA-G molecule in the pathogenesis and progression of immune-based diseases and infections, underlining the relevance of its investigation with the aim to developing new therapeutic strategies and clinical markers. Meanwhile, the analysis of the interactions between HLA-G and other HLA-Ib molecules may be useful to understand the mechanisms for the creation of immune-suppressive microenvironments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Elizabeth Jenkins for helpful corrections to the manuscript.
References
1. Carosella ED, Moreau P, Lemaoult J, Rouas-Freiss N. HLA-G: from biology to clinical benefits. Trends Immunol (2008) 29:125–32. doi: 10.1016/j.it.2007.11.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood (2008) 111:4862–70. doi:10.1182/blood-2007-12-127662
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Ishitani A, Gerarthy DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci U S A (1992) 89:3947–51. doi:10.1073/pnas.89.9.3947
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Paul P, Cabestre FA, Ibrahim EC, Lefevbre S, Khalil-Daher I, Vazeux G, et al. Identification of HLA-G7 as a new slice variant of the HLA-G mRNA and expression of soluble HL-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol (2000) 61:1138–49. doi:10.1016/S0198-8859(00)00197-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science (1990) 144:220–3. doi:10.1126/science.2326636
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Larsen MH, Hviid TV. Human leukocyte antigen-G polymorphism in relation to expression, function, and disease. Hum Immunol (2009) 70:1026–34. doi:10.1016/j.humimm.2009.07.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Hviid TV, Rizzo R, Christiansen OB, Melchiorri L, Lindhard A, Baricordi OR. HLA-G and IL-10 in plasma in relation to HLA-G genotype and polymorphisms. Immunogenetics (2004) 56:135–41. doi:10.1007/s00251-004-0673-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Yan WH, Lin A, Chen XJ, Dai MZ, Gan LH, Zhou MY, et al. Association of the maternal 14-bp insertion polymorphism in the HLA-G gene in women with recurrent spontaneous abortions. Tissue Antigens (2006) 68:521–3. doi:10.1111/j.1399-0039.2006.00723.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Hviid TV, Christiansen OB. Linkage disequilibrium between human leukocyte antigen (HLA) class II and HLA-G – possible implications for human reproduction and autoimmune disease. Hum Immunol (2005) 66:688–99. doi:10.1016/j.humimm.2005.03.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Veit TD, Chies JAB. Tolerance versus immune response microRNAs as important elements in the regulation of the HLA-G gene expression. Transpl Immunol (2009) 20:229–31. doi:10.1016/j.trim.2008.11.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. LeMaoult J, Le Discorde M, Rouas-Freiss N, Moreau P, Menier C, McCluskey J, et al. Biology and functions of human leukocyte antigen-G in health and sickness. Tissue Antigens (2003) 62:273–84. doi:10.1034/j.1399-0039.2003.00143.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Park GM, Lee S, Park B, Kim E, Shin J, Cho K, et al. Soluble HLA-G generated by proteolytic shedding inhibits NKmediated cell lysis. Biochem Biophys Res Commun (2004) 313:606–11. doi:10.1016/j.bbrc.2003.11.153
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Dong Y, Lieskovska J, Kedrin D, Porcelli S, Mandelboim O, Bushkin Y. Soluble nonclassical HLA generated by the metalloproteinase pathway. Hum Immunol (2003) 64:802–10. doi:10.1016/S0198-8859(03)00093-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Demaria S, Schwab R, Gottesman SRS, Bushkin Y. Soluble b2-microglobulin-free class I heavy chains are released from the surface of activated and leukemia cells by a metalloprotease. J Biol Chem (1994) 269:6689–94.
15. Zidi I, Guillard C, Marcou C, Krawice-Radanne I, Sangrouber D, Rouas-Freiss N, et al. Increase in HLA-G1 proteolytic shedding by tumor cells: a regulatory pathway controlled by NF-kappaB inducers. Cell Mol Life Sci (2006) 63:2669–81. doi:10.1007/s00018-006-6341-y
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Rizzo R, Trentini A, Bortolotti D, Manfrinato MC, Rotola A, Castellazzi M, et al. Matrix metalloproteinase-2 (MMP-2) generates soluble HLA-G1 by cell surface proteolytic shedding. Mol Cell Biochem (2013) 381:243–55. doi:10.1007/s11010-013-1708-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Clements CS, Kjer-Nielsen L, Kostenko L, Hoare HL, Dunstone MA, Moses E, et al. Crystal structure of HLA-G: a nonclassical MHC class I molecule expressed at the fetal-maternal interface. Proc Natl Acad Sci U S A (2005) 102:3360–5. doi:10.1073/pnas.0409676102
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Juch H, Blaschitz A, Daxböck C, Rueckert C, Kofler K, Dohr G. A novel sandwich ELISA for alpha1 domain based detection of soluble HLA-G heavy chains. J Immunol Methods (2005) 307:96–106. doi:10.1016/j.jim.2005.09.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Boyson JE, Erskine R, Whitman MC, Chiu M, Lau JM, Koopman LA, et al. Disulfide bond-mediated dimerization of HLA-G on the cell surface. Proc Natl Acad Sci U S A (2002) 99:16180–5. doi:10.1073/pnas.212643199
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Gonen-Gross T, Achdout H, Gazit R, Hanna J, Mizrahi S, Markel G, et al. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J Immunol (2003) 171:1343–51. doi:10.4049/jimmunol.171.3.1343
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Favier B, HoWangYin KY, Wu J, Caumartin J, Daouya M, Horuzsko A, et al. Tolerogenic function of dimeric forms of HLA-G recombinant proteins: a comparative study in vivo. PLoS One (2011) 6:e21011. doi:10.1371/journal.pone.0021011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol (2007) 37:1924–37. doi:10.1002/eji.200737089
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Zilberman S, Schenowitz C, Agaugué S, Benoît F, Riteau B, Rouzier R, et al. HLA-G1 and HLA-G5 active dimers are present in malignant cells and effusions: the influence of the tumor microenvironment. Eur J Immunol (2012) 42:1599–608. doi:10.1002/eji.201141761
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Ellis SA, Palmer LS, McMichael AJ. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA class I molecule. J Immunol (1990) 144:731–5.
25. Mallet V, Blaschitz A, Crisa L, Schmitt C, Fournel S, King A, et al. HLA-G in the human thymus: a subpopulation of medullary epithelial but not CD83(+) dendritic cells expresses HLA-G as a membrane-bound and soluble protein. Int Immunol (1999) 11:889–98. doi:10.1093/intimm/11.6.889
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Le Discorde M, Moreau P, Sabatier P, Legeais JM, Carosella ED. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum Immunol (2003) 64:1039–44. doi:10.1016/j.humimm.2003.08.346
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Ito T, Ito N, Saathoff M, Stampachiacchiere B, Bettermann A, Bulfone-Paus S, et al. Immunology of the human nail apparatus: the nail matrix is a site of relative immune privilege. J Invest Dermatol (2005) 125:1139–48. doi:10.1111/j.0022-202X.2005.23927.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Cirulli V, Zalatan J, McMaster M, Prinsen R, Salomon DR, Ricordi C, et al. The class I HLA repertoire of pancreatic islets comprises the nonclassical class Ib antigen HLA-G. Diabetes (2006) 55:1214–22. doi:10.2337/db05-0731
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Menier C, Rabreau M, Challier JC, Le Discorde M, Carosella ED, Rouas-Freiss N. Erythroblasts secrete the nonclassical HLA-G molecule from primitive to definitive hematopoiesis. Blood (2004) 104:3153–60. doi:10.1182/blood-2004-03-0809
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, et al. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol (1999) 11:803–11. doi:10.1093/intimm/11.5.803
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Rizzo R, Melchiorri L, Stignani M, Baricordi OR. HLA-G expression is a fundamental prerequisite to pregnancy. Hum Immunol (2007) 68:244–50. doi:10.1016/j.humimm.2006.10.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Lila N, Amrein C, Guillemain R, Chevalier P, Latremouille C, Fabiani JN, et al. Human leukocyte antigen-G expression after heart transplantation is associated with a reduced incidence of rejection. Circulation (2002) 105:1949–54. doi:10.1161/01.CIR.0000015075.89984.46
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Pistoia V, Morandi F, Wang X, Ferrone S. Soluble HLA-G: are they clinically relevant? Semin Cancer Biol (2007) 17:469–79. doi:10.1016/j.semcancer.2007.07.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Paul P, Rouas-Freiss N, Khalil-Daher I, Moreau P, Riteau B, Le Gal FA, et al. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci U S A (1998) 95:4510–5. doi:10.1073/pnas.95.8.4510
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Onno M, Pangault C, Le Friec G, Guilloux V, Andre P, Fauchet R. Modulation of HLA-G antigens expression by human cytomegalovirus: specific induction in activated macrophages harboring human cytomegalovirus infection. J Immunol (2000) 164:6426–34. doi:10.4049/jimmunol.164.12.6426
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Rouas-Freiss N, Marchal RE, Kirszenbaum M, Dausset J, Carosella ED. The α1 domain of HLA-G1 and HLA-G2 inhibits cytotoxicity induced by natural killer cells: is HLA-G the public ligand for natural killer cell inhibitory receptors? Proc Natl Acad Sci U S A (1997) 94:5249–54. doi:10.1073/pnas.94.10.5249
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Fournel S, Aguerre-Gi M, Huc X, Lenfant F, Alam A, Toubert A, et al. Cutting edge: soluble HLA-G1 triggers CD95/CD 95 ligand-mediated apoptosis in activated CD8+ cells by interacting CD8. J Immunol (2000) 164:6100–4. doi:10.4049/jimmunol.164.12.6100
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Contini P, Ghio M, Poggi A, Filaci G, Indiveri F, Ferrone S, et al. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol (2003) 33:125–34. doi:10.1002/immu.200390015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Lila N, Rouas-Freiss N, Dausset J, Carpentier A, Carosella ED. Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: a CD4+ T cell regulatory mechanism. Proc. Natl Acad Sci U S A (2001) 98:12150–5. doi:10.1073/pnas.201407398
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Le Rond S, Azèma C, Krawice-Radanne I, Durrbach A, Guettier C, Carosella ED, et al. Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/regulatory T cells. J Immunol (2006) 176:3266–76. doi:10.4049/jimmunol.176.5.3266
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Horuzsko A, Lenfant F, Munn DH, Mellor AL. Maturation of antigen-presenting cells is compromised in HLA-G transgenic mice. Int Immunol (2001) 13:385–94. doi:10.1093/intimm/13.3.385
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Colonna M, Navarro F, Bellón T, Llano M, García P, Samaridis J, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med (1997) 186:1809–18. doi:10.1084/jem.186.11.1809
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Kanai T, Fujii T, Kozuma S, Yamashita T, Miki A, Kikuchi A, et al. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol Hum Reprod (2001) 7:195–200. doi:10.1093/molehr/7.2.195
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood (2007) 109:2040–8. doi:10.1182/blood-2006-05-024547
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood (2010) 11:935–44. doi:10.1182/blood-2009-07-234872
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J (2005) 19:662–4. doi:10.1096/fj.04-1617fje
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
47. Carosella ED, Moreau P, Aractingi S, Rouas-Freiss N. HLA-G: a shield against inflammatory aggression. Trends Immunol (2001) 22:553–5. doi:10.1016/S1471-4906(01)02007-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
48. Rizzo R, Bortolotti D, Baricordi OR, Fainardi E. New insights into HLA-G and inflammatory diseases. Inflamm Allergy Drug Targets (2012) 11:448–63. doi:10.2174/187152812803590037
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Torres MI, López-Casado MA, Luque J, Peña J, Ríos A. New advances in coeliac disease: serum and intestinal expression of HLA-G. Int Immunol (2006) 18:713–8. doi:10.1093/intimm/dxl008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
50. Fabris A, Segat L, Catamo E, Morgutti M, Vendramin A, Crovella S. HLA-G 14 bp deletion/insertion polymorphism in celiac disease. Am J Gastroenterol (2011) 106:139–44. doi:10.1038/ajg.2010.340
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
51. Rizzo R, Melchiorri L, Simone L, Stignani M, Marzola A, Gullini S, et al. Different production of soluble HLA-G antigens by peripheral blood mononuclear cells in ulcerative colitis and Crohn’s disease: a noninvasive diagnostic tool? Inflamm Bowel Dis (2008) 14:100–5. doi:10.1002/ibd.20281
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Zelante A, Borgoni R, Galuppi C, Cifalà V, Melchiorri L, Gullini S, et al. Therapy modifies HLA-G secretion differently in Crohn’s disease and ulcerative colitis patients. Inflamm Bowel Dis (2011) 17:E94–5. doi:10.1002/ibd.21756
53. Lee HM, Sugino H, Aoki C, Shimaoka Y, Suzuki R, Ochi K, et al. Abnormal networks of immune response-related molecules in bone marrow cells from patients with rheumatoid arthritis as revealed by DNA microarray analysis. Arthritis Res Ther (2011) 13:R89. doi:10.1186/ar3364
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
54. Veit TD, Vianna P, Scheibel I, Brenol CV, Brenol JC, Xavier RM, et al. Association of the HLA-G 14-bp insertion/deletion polymorphism with juvenile idiopathic arthritis and rheumatoid arthritis. Tissue Antigens (2008) 71:440–6. doi:10.1111/j.1399-0039.2008.01019.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
55. Kim SK, Chung JH, Kim DH, Yun DH, Hong SJ, Lee KH. Lack of association between promoter polymorphisms of HLA-G gene and rheumatoid arthritis in Korean population. Rheumatol Int (2012) 32:509–12. doi:10.1007/s00296-010-1735-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
56. Catamo E, Addobbati C, Segat L, Sotero Fragoso T, Domingues Barbosa A, Tavares Dantas A, et al. HLA-G gene polymorphisms associated with susceptibility to rheumatoid arthritis disease and its severity in Brazilian patients. Tissue Antigens (2014) 84:308–15. doi:10.1111/tan.12396
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Verbruggen LA, Rebmann V, Demanet C, De Cock S, Grosse-Wilde H. Soluble HLA-G in rheumatoid arthritis. Hum Immunol (2006) 67:561–7. doi:10.1016/j.humimm.2006.03.023
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. Prigione I, Penco F, Martini A, Gattorno M, Pistoia V, Morandi F. HLA-G and HLA-E in patients with juvenile idiopathic arthritis. Rheumatology (2011) 50:966–72. doi:10.1093/rheumatology/keq418
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Ongaro A, Stignani M, Pellati A, Melchiorri L, Massari L, Caruso G, et al. Human leukocyte antigen-G molecules are constitutively expressed by synovial fibroblasts and upmodulated in osteoarthritis. Hum Immunol (2010) 71:342–50. doi:10.1016/j.humimm.2010.01.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
60. Rudwaleit M, Yin Z, Siegert S, Grolms M, Radbruch A, Braun J, et al. Response to methotrexate in early rheumatoid arthritis is associated with a decrease of T cell derived tumour necrosis factor alpha, increase of interleukin 10, and predicted by the initial concentration of interleukin 4. Ann Rheum Dis (2000) 59:311–4. doi:10.1136/ard.59.4.311
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
61. Rizzo R, Hviid TV, Stignani M, Balboni A, Grappa MT, Melchiorri L, et al. The HLA-G genotype is associated with IL-10 levels in activated PBMCs. Immunogenetics (2005) 57:172–81. doi:10.1007/s00251-005-0788-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
62. Rizzo R, Rubini M, Govoni M, Padovan M, Melchiorri L, Stignani M, et al. HLA-G 14-bp polymorphism regulates the methotrexate response in rheumatoid arthritis. Pharmacogenet Genomics (2006) 16:615–23. doi:10.1097/01.fpc.0000230115.41828.3a
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
63. Stamp LK, O’Donnell JL, Chapman PT, Barclay ML, Kennedy MA, Frampton CM, et al. Lack of association between HLA-G 14 bp insertion/deletion polymorphism and response to long-term therapy with methotrexate response in rheumatoid arthritis. Ann Rheum Dis (2009) 68:154–5. doi:10.1136/ard.2008.089383
64. Kooloos WM, Wessels JA, van der Straaten T, Allaart CF, Huizinga TW, Guchelaar HJ. Functional polymorphisms and methotrexate treatment outcome in recent-onset rheumatoid arthritis. Pharmacogenomics (2010) 11:163–75. doi:10.2217/pgs.09.139
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
66. Wastowski IJ, Sampaio-Barros PD, Amstalden EM, Palomino GM, Marques-Neto JF, Crispim JC, et al. HLA-G expression in the skin of patients with systemic sclerosis. J Rheumatol (2009) 36:1230–4. doi:10.3899/jrheum.080552
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
67. Rosado S, Perez-Chacon G, Mellor-Pita S, Sanchez-Vegazo I, Bellas-Menendez C, Citores MJ, et al. Expression of human leukocyte antigen-G in systemic lupus erythematosus. Hum Immunol (2008) 69:9–15. doi:10.1016/j.humimm.2007.11.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
68. Chen J, Shen B, Jiang Y, Jun L, Zhu M, Chen B, et al. Analysis of immunoglobulin-like transcripts (ILTs) in lymphocytes with sHLA-G and IL10 from SLE patients. Clin Exp Med (2013) 13:135–42. doi:10.1007/s10238-012-0185-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
69. Rizzo R, Hviid TV, Govoni M, Padovan M, Rubini M, Melchiorri L, et al. HLA-G genotype and HLA-G expression in systemic lupus erythematosus: HLA-G as a putative susceptibility gene in systemic lupus erythematosus. Tissue Antigens (2008) 71:520–9. doi:10.1111/j.1399-0039.2008.01037.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
70. Rudstein-Svetlicky N, Loewenthal R, Horejsi V, Gazit E. HLA-G levels in serum and plasma. Tissue Antigens (2007) 69(Suppl 1):140–2. doi:10.1111/j.1399-0039.2006.763_4.x
71. Monsiváis-Urenda AE, Baranda L, Alvarez-Quiroga C, Abud-Mendoza C, González-Amaro R. Expression and functional role of HLA-G in immune cells from patients with systemic lupus erythematosus. J Clin Immunol (2011) 31:369–78. doi:10.1007/s10875-010-9496-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
72. Fernando MM, Freudenberg J, Lee A, Morris DL, Boteva L, Rhodes B, et al. Transancestral mapping of the MHC region in systemic lupus erythematosus identifies new independent and interacting loci at MSH5, HLA-DPB1 and HLA-G. Ann Rheum Dis (2012) 71:777–84. doi:10.1136/annrheumdis-2011-200808
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
73. Veit TD, Cordero EA, Mucenic T, Monticielo OA, Brenol JC, Xavier RM, et al. Association of the HLA-G 14 bp polymorphism with systemic lupus erythematosus. Lupus (2009) 18:424–30. doi:10.1177/0961203308098187
74. Pedroza LS, Sauma MF, Vasconcelos JM, Takeshita LY, Ribeiro-Rodrigues EM, Sastre D, et al. Systemic lupus erythematosus: association with KIR and SLC11A1 polymorphisms, ethnic predisposition and influence in clinical manifestations at onset revealed by ancestry genetic markers in an urban Brazilian population. Lupus (2011) 20:265–73. doi:10.1177/0961203310385266
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
75. Lucena-Silva N, de Souza VS, Gomes RG, Fantinatti A, Muniz YC, de Albuquerque RS, et al. HLA-G 3’ untranslated region polymorphisms are associated with systemic lupus erythematosus in 2 Brazilian populations. J Rheumatol (2013) 40:1104–13. doi:10.3899/jrheum.120814
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
76. Consiglio CR, Veit TD, Monticielo OA, Mucenic T, Xavier RM, Brenol JC, et al. Association of the HLA-G gene +3142C >G polymorphism with systemic lupus erythematosus. Tissue Antigens (2011) 77:540–5. doi:10.1111/j.1399-0039.2011.01635.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
77. Park KS, Nam JH, Lee ES, Choi JS, Bang D, Lee S. Increased risk of human leukocyte antigen-G gene variants in Behçet’s disease. Clin Exp Rheumatol (2006) 24(5 Suppl 42):S126–7.
78. Park KS, Park JS, Nam JH, Bang D, Sohn S, Lee ES. HLA-E*0101 and HLA-G*010101 reduce the risk of Behcet’s disease. Tissue Antigens (2007) 69:139–44. doi:10.1111/j.1399-0039.2006.00742.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
79. Kim JJ, Hong SJ, Hong YM, Kim S, Kang MJ, Kim KJ, et al. Genetic variants in the HLA-G region are associated with Kawasaki disease. Hum Immunol (2008) 69:867–71. doi:10.1016/j.humimm.2008.10.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
80. Bos JD. Skin Immune System: Cutaneous Immunology and Clinical Immunodermatology. 3rd ed. Boca Raton, FL: CRC Press (2005). p. 77–99.
81. Ulbrecht M, Rehberger B, Strobel I, Messer G, Kind P, Degitz K, et al. HLA-G: expression in human keratinocytes in vitro and in human skin in vivo. Eur J Immunol (1994) 24:176–80. doi:10.1002/eji.1830240127
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
82. Cardili RN, Alves TG, Freitas JC, Soares CP, Mendes-Junior CT, Soares EG, et al. Expression of human leucocyte antigen-G primarily targets affected skin of patients with psoriasis. Br J Dermatol (2010) 63(4):769–75. doi:10.1111/j.1365-2133.2010.09917.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
83. Urosevic M, Kamarashev J, Burg G, Dummer R. Primary cutaneous CD8+ and CD56+ T-cell lymphomas express HLA-G and killer cell inhibitory ligand, ILT2. Blood (2004) 103:1796–8. doi:10.1182/blood-2003-10-3372
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
84. Urosevic M, Kempf W, Zagrodnik B, Panizzon R, Burg G, Dummer R. HLA-G expression in basal cell carcinomas of the skin recurring after radiotherapy. Clin Exp Dermatol (2005) 30:422–5. doi:10.1111/j.1365-2230.2005.01790.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
85. Urosevic M, Willers J, Mueller B, Kempf W, Burg G, Dummer R. HLAG protein up-regulation in primary cutaneous lymphomas is associated with IL-10 expression in large cell T-cell lymphomas and indolent B-cell lymphomas. Blood (2002) 99:609–17. doi:10.1182/blood.V99.2.609
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
86. Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med (1999) 341:1817–28. doi:10.1056/NEJM199912093412407
87. Sweeney C, Kirby B. Does HLA-G prevent tissue destruction in psoriasis? Br J Dermatol (2011) 164:1118–9. doi:10.1111/j.1365-2133.2011.10222.x
88. Borghi A, Fogli E, Stignani M, Melchiorri L, Altieri E, Baricordi OR, et al. Soluble human leukocyte antigen-G and interleukin-10 levels in plasma of psoriatic patients: preliminary study on a possible correlation between generalized immune status, treatments and disease. Arch Dermatol Res (2008) 300:551–9. doi:10.1007/s00403-008-0886-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
89. Borghi A, Rizzo R, Corazza M, Bertoldi AM, Bortolotti D, Sturabotti G, et al. HLA-G 14-bp polymorphism: a possible marker of systemic treatment response in psoriasis vulgaris? Preliminary results of a retrospective study. Dermatol Ther (2014) 27:284–9. doi:10.1111/dth.12140
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
90. Yari F, Zavaran Hosseini A, Nemat Gorgani M, Khorramizadeh MR, Mansouri P, Kazemnejad A. Expression of HLA-G in the skin of patients with pemphigus vulgaris. Iran J Allergy Asthma Immunol (2008) 7:7–12. doi:07.01/ijaai.712
91. Gazit E, Slomov Y, Goldberg I, Brenner S, Loewenthal R. HLA-G is associated with pemphigus vulgaris in Jewish patients. Hum Immunol (2004) 65:39–46. doi:10.1016/j.humimm.2003.09.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
92. Solini A, Muscelli E, Stignani M, Melchiorri L, Santini E, Rossi C, et al. Soluble human leukocyte antigen-g expression and glucose tolerance in subjects with different degrees of adiposity. J Clin Endocrinol Metab (2010) 95:3342–6. doi:10.1210/jc.2009-2747
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
93. Eike MC, Becker T, Humphreys K, Olsson M, Lie BA. Conditional analyses on the T1DGC MHC dataset: novel associations with type 1 diabetes around HLA-G and confirmation of HLA-B. Genes Immun (2009) 10:56–67. doi:10.1038/gene.2008.74
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
94. Abediankenari S, Eslami MB, Sarrafnejad A, Mohseni M, Larijani B. Dendritic cells bearing HLA-G inhibit T-Cell activation in type 1 diabetes. Iran J Allergy Asthma Immunol (2007) 6:1–7.
95. García-González IJ, Valle Y, Rivas F, Figuera-Villanueva LE, Muñoz-Valle JF, Flores-Salinas HE, et al. The 14 bp Del/Ins HLA-G polymorphism is related with high blood pressure in acute coronary syndrome and type 2 diabetes mellitus. Biomed Res Int (2014) 2014:898159. doi:10.1155/2014/898159
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
96. Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol (2009) 9:393–407. doi:10.1038/nri2550
97. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med (2000) 43:938–52. doi:10.1056/NEJM200009283431307
98. Compston A, Coles A. Multiple sclerosis. Lancet (2008) 372:1502–17. doi:10.1016/S0140-6736(08)61620-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
99. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol (2011) 69:292–302. doi:10.1002/ana.22366
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
100. Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest (2012) 122:1180–8. doi:10.1172/JCI58649
101. Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron (2006) 52:61–76. doi:10.1016/j.neuron.2006.09.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
102. Fainardi E, Rizzo R, Castellazzi M, Stignani M, Granieri E, Baricordi OR. Potential role of soluble human leukocyte antigen-G molecules in multiple sclerosis. Hum Immunol (2009) 70:981–7. doi:10.1016/j.humimm.2009.07.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
103. Fainardi E, Castellazzi M, Stignani M, Morandi F, Sana G, Gonzalez R, et al. Emerging topics and new perspectives on HLA-G. Cell Mol Life Sci (2011) 68:433–51. doi:10.1007/s00018-010-0584-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
104. Fainardi E, Rizzo R, Melchiorri L, Vaghi L, Castellazzi M, Marzola A, et al. Presence of detectable levels of soluble HLA-G molecules in CSF of relapsing-remitting multiple sclerosis: relationship with CSF soluble HLA-I and IL-10 concentrations and MRI findings. J Neuroimmunol (2003) 142:149–58. doi:10.1016/S0165-5728(03)00266-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
105. Fainardi E, Rizzo R, Melchiorri L, Castellazzi M, Paolino E, Tola MR, et al. Intrathecal synthesis of soluble HLA-G and HLA-I molecules are reciprocally associated to clinical and MRI activity in patients with multiple sclerosis. Mult Scler (2006) 12:2–12. doi:10.1191/1352458506ms1241oa
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
106. Fainardi E, Rizzo R, Melchiorri L, Stignani M, Castellazzi M, Caniatti ML, et al. Soluble HLA-G molecules are released as HLA-G5 and not as soluble HLA-G1 isoforms in CSF of patients with relapsing-remitting multiple sclerosis. J Neuroimmunol (2007) 192:219–25. doi:10.1016/j.jneuroim.2007.10.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
107. Fainardi E, Rizzo R, Melchiorri L, Stignani M, Castellazzi M, Tamborino C, et al. CSF levels of soluble HLA-G and Fas molecules are inversely associated to MRI evidence of disease activity in patients with relapsing remitting multiple sclerosis. Mult Scler (2008) 14:446–54. doi:10.1177/1352458507085137
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
108. Mitsdoerffer M, Schreiner B, Kieseier B, Neuhaus O, Dichgans J, Hartung H-P, et al. Monocyte-derived HLA-G acts as a strong inhibitor of autologous CD4 T cell activation and is upregulated by interferon-β in vitro and in vivo: rationale for the therapy of multiple sclerosis. J Neuroimmunol (2005) 159:155–64. doi:10.1016/j.jneuroim.2004.09.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
109. Airas L, Nikula T, Huang Y-H, Lahesmaa R, Wiendl H. Post-partum-activation of multiple sclerosis is associated with down-regulation of tolerogenic HLA-G. J Neuroimmunol (2007) 187:205–11. doi:10.1016/j.jneuroim.2007.05.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
110. Wiendl H, Feger U, Mittelbronn M, Jack C, Schreiner B, Stadelmann C, et al. Expression of the immune-tolerogenic major histocompatibility molecule HLA-G in multiple sclerosis: implications for CNS immunity. Brain (2005) 128:2689–704. doi:10.1093/brain/awh609
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
111. Feger U, Tolosa E, Huang Y-H, Waschbisch A, Biedermann T, Melms A, et al. HLA-G expression defines a novel regulatory T cell subset present in human peripheral blood and sites of inflammation. Blood (2007) 110:568–77. doi:10.1182/blood-2006-11-057125
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
112. Huang YH, Zozulya AL, Weidenfeller C, Schwab N, Wiendl H. T cell suppression by naturally occurring HLA-G-expressing regulatory CD4+ T cells is IL-10-dependent and reversible. J Leukoc Biol (2009) 86:273–81. doi:10.1189/jlb.1008649
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
113. Huang YH, Zozulya AL, Weidenfeller C, Metz I, Buck D, Toyka KV, et al. Specific central nervous system recruitment of HLA-G (+) regulatory T cells in multiple sclerosis. Ann Neurol (2009) 66:171–83. doi:10.1002/ana.21705
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
114. Cree BA, Rioux JD, McCauley JL, Gourraud PA, Goyette P, McElroy J, et al. A major histocompatibility class I locus contributes to multiple sclerosis susceptibility independently from HLA-DRB1*15:01. PLoS One (2010) 5:e11296. doi:10.1371/journal.pone.0011296
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
115. Kroner A, Grimm A, Johannssen K, Mäurer M, Wiendl H. The genetic influence of the nonclassical MHC molecule HLA-G on multiple sclerosis. Hum Immunol (2007) 68:422–5. doi:10.1016/j.humimm.2007.01.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
116. Wisniewski A, Bilinska M, Klimczak A, Wagner M, Majorczyk E, Nowak I, et al. Association of the HLA-G gene polymorphism with multiple sclerosis in a polish population. Int J Immunogenet (2010) 37:307–11. doi:10.1111/j.1744-313X.2010.00926.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
117. Rizzo R, Bortolotti D, Fredj NB, Rotola A, Cura F, Castellazzi M, et al. Role of HLA-G 14bp deletion/insertion and +3142C >G polymorphisms in the production of sHLA-G molecules in relapsing-remitting multiple sclerosis. Hum Immunol (2012) 73:1140–6. doi:10.1016/j.humimm.2012.08.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
118. Fainardi E, Rizzo R, Melchiorri L, Castellazzi M, Govoni V, Caniatti L, et al. Beneficial effect of interferon-β 1b treatment in patients with relapsing-remitting multiple sclerosis is associated with an increase in serum levels of soluble HLA-I molecules during the first 3 months of therapy. J Neuroimmunol (2004) 148:206–11. doi:10.1016/j.jneuroim.2003.12.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
119. Waschbisch A, Sandbrink R, Hartung HP, Kappos L, Schwab S, Pohl C, et al. Evaluation of soluble HLA-G as a biomarker for multiple sclerosis. Neurology (2011) 77:596–8. doi:10.1212/WNL.0b013e318228c14d
120. Alcami A, Koszinowski UH. Viral mechanisms of immune evasion. Trends Microbiol (2000) 8:410–8. doi:10.1016/S0966-842X(00)01830-8
121. Tripathi P, Agrawal S. The role of human leukocyte antigen E and G in HIV infection. AIDS (2007) 21:1395–404. doi:10.1097/QAD.0b013e32810c8bbc
122. Lozano JM, Gonzalez R, Kindelan JM, Rouas-Freiss N, Caballos R, Dausset J, et al. Monocytes and T-lymphocytes in HIV-1-positive patients express HLA-G molecule. AIDS (2002) 16:347–51. doi:10.1097/00002030-200202150-00005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
123. Cabello A, Rivero A, Garcia MJ, Lozano JM, Torre-Cisneros J, Gonzalez R, et al. HAART induces the expression of HLA-G on peripheral monocytes in HIV-1 infected individuals. Hum Immunol (2003) 64:1045–9. doi:10.1016/j.humimm.2003.08.353
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
124. Li C, Toth I, Schulze Zur Wiesch J, Pereyra F, Rychert J, Rosenberg ES, et al. Functional characterization of HLA-G+ regulatory T cells in HIV-1 infection. PLoS Pathog (2013) 9:e1003140. doi:10.1371/journal.ppat.1003140
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
125. da Silva GK, Vianna P, Veit TD, Crovella S, Catamo E, Cordero EA, et al. Influence of HLA-G polymorphisms in human immunodeficiency virus infection and hepatitis C virus co-infection in Brazilian and Italian individuals. Infect Genet Evol (2014) 21:418–23. doi:10.1016/j.meegid.2013.12.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
126. Segat L, Zupin L, Kim HY, Catamo E, Thea DM, Kankasa C, et al. HLA-G 14bp deletion/insertion polymorphism and mother-to-child transmission of HIV. Tissue Antigens (2014) 83:161–7. doi:10.1111/tan.12296
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
127. Soderberg-Naucler C, Nelson JY. Human cytomegalovirus latency and reactivation – a delicate balance between the virus and its host’s immune system. Intervirology (1999) 42:314–21. doi:10.1159/000053966
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
128. Yan WH, Lin A, Chen BG, Chen SY. Induction of both membrane-bound and soluble HLA-G expression in active human cytomegalovirus infection. J Infect Dis (2009) 200:820–6. doi:10.1086/604733
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
129. Spencer JV, Lockridge KM, Barry PA, Lin G, Tsang M, Penfold ME, et al. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol (2002) 76:1285–92. doi:10.1128/JVI.76.3.1285-1292.2002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
130. Xu HH, Shi WW, Lin A, Yan WH. HLA-G 3’ untranslated region polymorphisms influence the susceptibility for human papillomavirus infection. Tissue Antigens (2014) 84(2):216–22. doi:10.1111/tan.12359
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
131. Smith MA, Tellier PP, Roger M, Coutlée F, Franco EL, Richardson H. Determinants of human papillomavirus coinfections among Montreal university students: the influence of behavioral and biologic factors. Cancer Epidemiol Biomarkers Prev (2014) 23(5):812–22. doi:10.1158/1055-9965.EPI-13-1255
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
132. Metcalfe S, Roger M, Faucher MC, Coutlée F, Franco EL, Brassard P. The association between human leukocyte antigen (HLA)-G polymorphisms and human papillomavirus (HPV) infection in Inuit women of northern Quebec. Hum Immunol (2013) 74(12):1610–5. doi:10.1016/j.humimm.2013.08.279
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
133. Silva ID, Muniz YC, Sousa MC, Silva KR, Castelli EC, Filho JC, et al. HLA-G 3’UTR polymorphisms in high grade and invasive cervico-vaginal cancer. Hum Immunol (2013) 74(4):452–8. doi:10.1016/j.humimm.2012.11.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
134. Dong DD, Yang H, Li K, Xu G, Song LH, Fan XL, et al. Human leukocyte antigen-G (HLA-G) expression in cervical lesions: association with cancer progression, HPV 16/18 infection, and host immune response. Reprod Sci (2010) 17(8):718–23. doi:10.1177/1933719110369183
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
135. Simões RT, Gonçalves MA, Castelli EC, Júnior CM, Bettini JS, Discorde ML, et al. HLA-G polymorphisms in women with squamous intraepithelial lesions harboring human papillomavirus. Mod Pathol (2009) 22(8):1075–82. doi:10.1038/modpathol.2009.67
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
136. Guimarães MC, Soares CP, Donadi EA, Derchain SF, Andrade LA, Silva TG, et al. Low expression of human histocompatibility soluble leukocyte antigen-G (HLA-G5) in invasive cervical cancer with and without metastasis, associated with papilloma virus (HPV). J Histochem Cytochem (2010) 58(5):405–11. doi:10.1369/jhc.2009.954131
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
137. Yang YC, Chang TY, Chen TC, Lin WS, Chang SC, Lee YJ. Human leucocyte antigen-G polymorphisms are associated with cervical squamous cell carcinoma risk in Taiwanese women. Eur J Cancer (2014) 50(2):469–74. doi:10.1016/j.ejca.2013.10.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
138. Bortolotti D, Gentili V, Rotola A, Di Luca D, Rizzo R. Implication of HLA-G 3’ untranslated region polymorphisms in human papillomavirus infection. Tissue Antigens (2014) 83(2):113–8. doi:10.1111/tan.12281
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
139. Rodríguez JA, Galeano L, Palacios DM, Gómez C, Serrano ML, Bravo MM, et al. Altered HLA class I and HLA-G expression is associated with IL-10 expression in patients with cervical cancer. Pathobiology (2012) 79:72–83. doi:10.1159/000334089
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
140. Gillio-Tos A, Bicalho Mda G, Fiano V, Grasso C, Tarallo V, De Marco L, et al. Case-control study of HLA-G promoter methylation status, HPV infection and cervical neoplasia in Curitiba, Brazil: a pilot analysis. BMC Cancer (2012) 12:618. doi:10.1186/1471-2407-12-618
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
141. Rizzo R, Malagutti N, Bortolotti D, Gentili V, Rotola A, Fainardi E, et al. Infection and HLA-G molecules in nasal polyposis. J Immunol Res (2014) 2014:407430. doi:10.1155/2014/407430
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
142. Mégret F, Prehaud C, Lafage M, Moreau P, Rouas-Freiss N, Carosella ED, et al. Modulation of HLA-G and HLA-E expression in human neuronal cells after rabies virus or herpes virus simplex type 1 infections. Hum Immunol (2007) 68:294–302. doi:10.1016/j.humimm.2006.12.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
143. Cordero EA, Veit TD, da Silva MA, Jacques SM, Silla LM, Chies JA. HLA-G polymorphism influences the susceptibility to HCV infection in sickle cell disease patients. Tissue Antigens (2009) 74:308–13. doi:10.1111/j.1399-0039.2009.01331.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
144. Weng PJ, Fu YM, Ding SX, Xu DP, Lin A, Yan WH. Elevation of plasma soluble human leukocyte antigen-G in patients with chronic hepatitis C virus infection. Hum Immunol (2011) 72:406–11. doi:10.1016/j.humimm.2011.02.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
145. Park Y, Lim HS, Kim YS, Hong DJ, Kim HS. Soluble human leukocyte antigen-G expression in hepatitis B virus infection and hepatocellular carcinoma. Tissue Antigens (2012) 79:97–103. doi:10.1111/j.1399-0039.2011.01814.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
146. Shi WW, Lin A, Xu DP, Bao WG, Zhang JG, Chen SY, et al. Plasma soluble human leukocyte antigen-G expression is a potential clinical biomarker in patients with hepatitis B virus infection. Hum Immunol (2011) 72:1068–73. doi:10.1016/j.humimm.2011.06.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
147. Han Q, Li N, Zhu Q, Li Z, Zhang G, Chen J, et al. Association of serum soluble human leukocyte antigen-G levels with chronic hepatitis B virus infection. Clin Exp Med (2014) 14:35–43. doi:10.1007/s10238-012-0214-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
148. Amiot L, Vu N, Rauch M, L’Helgoualc’h A, Chalmel F, Gascan H, et al. Expression of HLA-G by mast cells is associated with hepatitis C virus-induced liver fibrosis. J Hepatol (2014) 60:245–52. doi:10.1016/j.jhep.2013.09.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
149. Koller BH, Geraghty DE, Shimizu Y, DeMars R, Orr HT. HLA-E. A novel HLA class I gene expressed in resting T lymphocytes. J Immunol (1988) 141:897–904.
150. Geraghty DE, Wei XH, Orr HT, Koller BH. Human leukocyte antigen F (HLA-F). An expressed HLA gene composed of a class I coding sequence linked to a novel transcribed repetitive element. J Exp Med (1990) 171:1–18. doi:10.1084/jem.171.1.1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
151. Sullivan LC, Clements CS, Rossjohn J, Brooks AG. The major histocompatibility complex class Ib molecule HLA-E at the interface between innate and adaptive immunity. Tissue Antigens (2008) 72:415–24. doi:10.1111/j.1399-0039.2008.01138.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
152. Garcia P, Llano M, de Heredia AB, Willberg CB, Caparrós E, Aparicio P, et al. Human T cell receptor-mediated recognition of HLA-E. Eur J Immunol (2002) 32:936–44. doi:10.1002/1521-4141(200204)32:4<936::AID-IMMU936>3.3.CO;2-D
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
153. Braud VM, Allan DS, O’Callaghan CA, Söderström K, D’Andrea A, Ogg GS, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature (1998) 391:795–9. doi:10.1038/35869
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
154. Carosella ED, Paul P, Moreau P, Rouas-Freiss N. HLA-G and HLA-E: fundamental and pathophysiological aspects. Immunol Today (2000) 21:532–4. doi:10.1016/S0167-5699(00)01707-2
155. Lee N, Ishitani A, Geraght DE. HLA-F is a surface marker on activated lymphocytes,”. Eur J Immunol (2010) 40:2308–18. doi:10.1002/eji.201040348
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
156. Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, et al. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol (2003) 171:1376–84. doi:10.4049/jimmunol.171.3.1376
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
157. Fiszer D, Ulbrecht M, Fernandez N, Johnson JP, Weiss EH, Kurpisz M. Analysis of HLA class Ib gene expression in male gametogenic cells. Eur J Immunol (1997) 27:1691–5. doi:10.1002/eji.1830270715
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
158. Zhang X, Lin A, Zhang JG, Bao WG, Xu DP, Ruan YY, et al. Alteration of HLA-F and HLA I antigen expression in the tumor is associated with survival in patients with esophageal squamous cell carcinoma. Int J Cancer (2013) 132:82–9. doi:10.1002/ijc.27621
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
159. Morandi F, Pistoia V. Interactions between HLA-G and HLA-E in physiological and pathological conditions. Front Immunol (2014) 22(5):394. doi:10.3389/fimmu.2014.00394
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
160. Morandi F, Cangemi G, Barco S, Amoroso L, Giuliano M, Gigliotti AR, et al. Plasma levels of soluble HLA-E and HLA-F at diagnosis may predict overall survival of neuroblastoma patients. Biomed Res Int (2013) 2013:956878. doi:10.1155/2013/956878
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
161. Morandi F, Venturi C, Rizzo R, Castellazzi M, Baldi E, Caniatti ML, et al. Intrathecal soluble HLA-E correlates with disease activity in patients with multiple sclerosis and may cooperate with soluble HLA-G in the resolution of neuroinflammation. J Neuroimmune Pharmacol (2013) 8:944–55. doi:10.1007/s11481-013-9459-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: HLA-G, inflammation, autoimmunity, infection, regulation
Citation: Rizzo R, Bortolotti D, Bolzani S and Fainardi E (2014) HLA-G molecules in autoimmune diseases and infections. Front. Immunol. 5:592. doi: 10.3389/fimmu.2014.00592
Received: 06 August 2014; Paper pending published: 11 October 2014;
Accepted: 04 November 2014; Published online: 18 November 2014.
Edited by:
Silvia Gregori, San Raffaele Telethon Institute for Gene Therapy, ItalyReviewed by:
Mepur Hanumantha-Rao Ravindranath, Terasaki Foundation Laboratory, USAErick C. Castelli, Universidade Estadual Paulista, Brazil
Copyright: © 2014 Rizzo, Bortolotti, Bolzani and Fainardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberta Rizzo, Department of Medical Sciences, Section of Microbiology and Medical Genetics, University of Ferrara, Via Luigi Borsari, 46, Ferrara 44121, Italy e-mail: rbr@unife.it
 Roberta Rizzo
Roberta Rizzo Daria Bortolotti
Daria Bortolotti Silvia Bolzani1
Silvia Bolzani1 Enrico Fainardi
Enrico Fainardi