Genetic Control of Ascorbic Acid Biosynthesis and Recycling in Horticultural Crops
- 1Group of Biotechnology of Pharmaceutical Plants, Laboratory of Pharmacognosy, Department of Pharmaceutical Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 2Laboratory of Agricultural Chemistry, Department of Crop Science, School of Agriculture, Aristotle University of Thessaloniki, Thessaloniki, Greece
Ascorbic acid (AsA) is an essential compound present in almost all living organisms that has important functions in several aspects of plant growth and development, hormone signaling, as well as stress defense networks. In recent years, the genetic regulation of AsA metabolic pathways has received much attention due to its beneficial role in human diet. Despite the great variability within species, genotypes, tissues and developmental stages, AsA accumulation is considered to be controlled by the fine orchestration of net biosynthesis, recycling, degradation/oxidation, and/or intercellular and intracellular transport. To date, several structural genes from the AsA metabolic pathways and transcription factors are considered to significantly affect AsA in plant tissues, either at the level of activity, transcription or translation via feedback inhibition. Yet, all the emerging studies support the notion that the steps proceeding through GDP-L-galactose phosphorylase and to a lesser extent through GDP-D-mannose-3,5-epimerase are control points in governing AsA pool size in several species. In this mini review, we discuss the current consensus of the genetic regulation of AsA biosynthesis and recycling, with a focus on horticultural crops. The aspects of AsA degradation and transport are not discussed herein. Novel insights of how this multifaceted trait is regulated are critical to prioritize candidate genes for follow-up studies toward improving the nutritional value of fruits and vegetables.
Introduction
Ascorbic acid (AsA) or vitamin C is one of the most abundant water-soluble low molecular weight antioxidants found throughout the plant cells including the apoplast exerting a central role in regulating the cellular redox potential (Sanmartin et al., 2003; Fotopoulos et al., 2006, 2008; Foyer and Noctor, 2011; Fotopoulos and Kanellis, 2013; Gest et al., 2013). As humans and some other primates lack the ability to synthesize and store AsA, they depend on fresh fruits and vegetables to cover their daily requirements. All recent studies build a strong case toward a diet rich in AsA for improving human health (Troesch et al., 2012), suggesting that AsA should be a clear target for the nutritional enhancement of horticultural crops. Due to its remarkable functions in plant growth and development, as well as its benefits in human diet, AsA regulation in plant edible organs has received much attention in recent years.
The accumulation of AsA within the same species may vary between different cultivars (Bulley et al., 2009; Mellidou et al., 2012a,b; Gest et al., 2013), tissue types (Bulley et al., 2009), and developmental stages (Pateraki et al., 2004; Bulley et al., 2009; Ioannidi et al., 2009; Mellidou et al., 2012b). In spite of this variability, AsA is tightly regulated by the fine orchestration of net biosynthesis, recycling, degradation/oxidation, and/or intercellular and intracellular transport. Several transgenic approaches have been employed to enhance AsA accumulation in plants, involving primarily leaf material of model plants (Arabidopsis or tobacco). Nevertheless, overexpression of several structural AsA-related genes from various AsA metabolic pathways had so far limited success in most species, raising concerns on the significance of single structural genes in the control of AsA concentrations. In this regard, it may be required to interfere into entire regulatory networks using more than one structural genes or transcription factors (TFs) in order to enrich AsA levels beyond the current levels. Although numerous studies on genetic factors indicated that AsA accumulation showed a relatively high heritability (Davey et al., 2006; Mellidou et al., 2012a; Bulley and Laing, 2016), the expression of certain transcripts critical to AsA metabolic pathways has also been shown to respond to environmental stimuli such as alterations in light density, temperature, ethylene, low oxygen and wounding (Sanmartin et al., 2007; Yabuta et al., 2007; Ioannidi et al., 2009; Massot et al., 2012; Yoshimura et al., 2014). On the basis of these considerations, this mini review shall attempt to illustrate the genetic factors governing the AsA pool in plant tissues with a focus on horticultural crops.
Genetic Regulation Through Promoting Biosynthesis
In higher plants, the current consensus is that AsA biosynthesis from glucose via the so-called L-galactose pathway (Figure 1; Wheeler et al., 1998, 2015) is the dominant route for AsA accumulation. Although conclusive evidence for all the intermediate steps has only relatively recently become available with the fully characterization of the Arabidopsis AsA-deficient mutants (Conklin et al., 2006; Laing et al., 2007), several structural genes from this pathway have been proposed to be key regulators of AsA concentrations in various species. A plethora of successful and less successful biotechnological approaches has been employed to enhance the AsA pool size, overcoming specific rate-limiting steps of the L-galactose pathway. The early, non-specific for AsA synthesis, genes of the main pathway, such as phosphomannose isomerase (PMI) and phosphomanno mutase (PMM), are less possible to exert a major control over AsA homeostasis (Qian et al., 2007; Maruta et al., 2008). The role of the next two genes of the L-galactose pathway, named GDP-D-mannose pyrophosphorylase (VTC1 or GMP) and GDP-D-mannose-3,5-epimerase (GME), is highly controversial. In the first case, expression of GMP has been correlated with AsA concentrations in some species, including acerola (Badejo et al., 2008), but not in tomato (Ioannidi et al., 2009), kiwifruit (Bulley et al., 2009), or blueberry (Liu et al., 2015). As GMP is also involved in cell wall polysaccharides synthesis and glycoproteins (Smirnoff, 2000), genes upstream this step are not solely committed to AsA biosynthesis. In the second case, a good correlation between GME transcripts and AsA had been reported in apple (Li et al., 2010b) and blueberry (Liu et al., 2015), but not in peach (Imai et al., 2009), tomato (Ioannidi et al., 2009; Mellidou et al., 2012b), or kiwifruit (Bulley et al., 2009). Modification of GME expression had only little effect on AsA pool of either leaf or fruit tissues (Bulley et al., 2009; Gilbert et al., 2009; Zhang et al., 2010; Mounet-Gilbert et al., 2016). Despite the fact that GME is not the rate-limiting step in AsA biosynthesis, it represents the intersection between the L-galactose pathway for the synthesis of AsA and the generation of monomers for cell wall biosynthesis, and it further seem to have distinct roles in pollen development, seed production, and vegetative growth (Mounet-Gilbert et al., 2016).
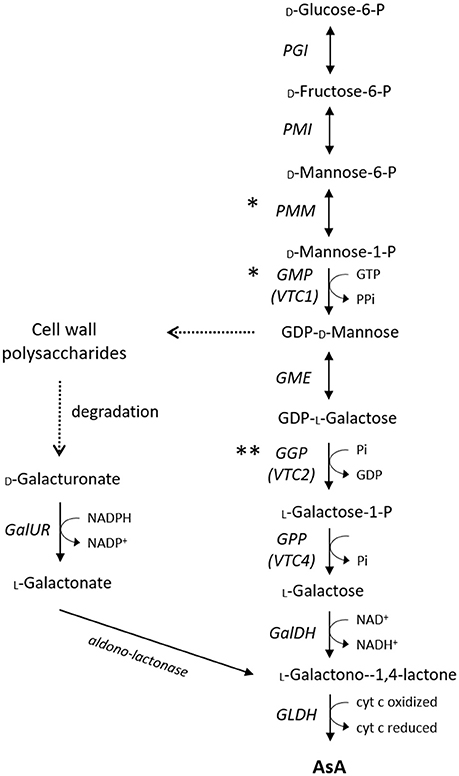
Figure 1. Major AsA biosynthetic pathways in plants. Asterisks indicate how transcription factors may influence AsA biosynthesis other than regulating gene transcription (*enzyme activity, ** translation). The cut arrows indicate simplified reactions with missing steps. PGI, Phosphoglucose Isomerase; PMI, Mannose-6-phosphate isomerase; PMM, Phosphomannomutase; GMP, GDP-D-mannose pyrophosphorylase; GME, GDP-D-mannose 3′ 5′ epimerase; GGP, GDP-L-galactose-phosphorylase; GPP, L-galactose-1-P phosphatase; GalDH, L-galactose dehydrogenase; GLDH, L-galactono-1,4-lactone dehydrogenase; GalUR, D-galacturonate reductase.
As GME catalyzes the double epimerization between L-galactose and L-gulose, a side branch from the main route has been reported to operate independently in terms of both enzyme specificity and subcellular location (Wolucka and Van Montagu, 2003), although its relevant contribution to AsA biosynthesis remains unknown. Beyond the ethical concerns for both humans and the environment, overexpression of the rat L-gulonolactone oxidase (GULO), the terminal enzyme of this “gulose shunt” significantly increased the AsA levels of several species (Jain and Nessler, 2000; Lim et al., 2012). Recently, GULO has been found to be functionally replaced with L-galactono-1,4-lactone dehydrogenase (GLDH) in photosynthetic eukaryotes in order to uncouple AsA biosynthesis from the generation of hydrogen peroxide with the acquisition of plastids through evolution (Wheeler et al., 2015).
Concerning the first committed step of the pathway, catalyzed by GDP-L-galactose phosphorylase (GGP), early studies on the ozone-sensitive AsA-deficient vtc2 Arabidopsis mutants (Conklin et al., 2000) showed that these mutants only contain 10–20% of the wild-type AsA levels resulting in insignificantly reduced growth. Recently, the presence of an independent cryptic mutation in GGP caused a similar reduction in AsA levels accompanied, however, by a smaller decrease in plant growth (Lim B. et al., 2016), pointing to a re-evaluation of GGP function in plants. Yet, several lines of evidence suggest GGP as the control point in the AsA biosynthetic pathway in several species including Arabidopsis (Bulley et al., 2009; Yoshimura et al., 2014), tobacco (Bulley et al., 2009; Wang et al., 2014), tomato (Bulley et al., 2012; Mellidou et al., 2012b; Wang L.-Y. et al., 2013), kiwifruit (Li et al., 2010c), citrus (Alós et al., 2013), blueberry (Liu et al., 2015), strawberry and potato tubers (Bulley et al., 2012). To a certain extent, this is fairly expected as GGP is placed at the first committed step of the pathway (Bulley and Laing, 2016). Further support to the pivotal role of GGP in AsA regulation arises from the fact that only GGP transcript levels significantly correlated with changes in AsA levels between high-AsA and low-AsA species in tomato (Mellidou et al., 2012b) and kiwifruit (Li et al., 2014). QTL studies in apple also provided strong evidence that GGP is the only structural gene tightly linked to flesh AsA levels independently of the environmental conditions (Mellidou et al., 2012a). Transient transformation using the kiwi or Arabidopsis genes revealed that GGP and GME operates synergistically to govern the AsA pool size (Bulley et al., 2009, 2012; Laing et al., 2015). Notably, when the transcript levels of either GGP or GME are modified (Gilbert et al., 2009; Zhang et al., 2010; Wang L.-Y. et al., 2013), the expression of the other one is also changed presumably to keep the balance in AsA biosynthesis and to maintain a stable AsA pool. Transcript levels of both genes are tightly regulated by light showing a diurnal trend (Dowdle et al., 2007; Massot et al., 2012). Specifically for GGP, its expression is culminated in the morning to support biosynthesis later on when maximum light density demands higher AsA levels (Dowdle et al., 2007). Allelic associations studies in apple reinforce the notion that SNPs found in GGP coding sequence are rather linked to polymorphisms in the promoter region that alter allele expression, than to altered protein function (Mellidou et al., 2012a).
Although the transcriptional control of GGP in regulating AsA accumulation is well recorded, it is only recently that its translational regulation has been elucidated under challenging conditions (Laing et al., 2015). The 5′-untranslated region (UTR) of GGP appears to contain a novel, highly conserved, non-canonical upstream open reading frame (uORF) in a wide range of species. Based on this observation, a model that allows feedback responsive regulation of AsA synthesis under unfavorable conditions has been proposed. According to this model, the uORF is translated and inhibits GGP translation at high AsA levels, while the uORF is disabled and GGP is translated at low AsA levels. The involvement of uORF in the regulation of GGP translation under rapidly altering conditions, without the need of gene transcription modification points out to a more robust way to control AsA concentrations. However, what merits further investigation is whether this conserved uORF and mutations within are able to explain the increased AsA levels found in some species or whether there are other factors synergistically or separately regulate GGP translation.
Despite the fact that expression patterns of the next gene of the pathway, namely L-galactose-1-P phosphatase (GPP or VTC4), were found to correlate with AsA concentrations in tomato and apple fruit (Ioannidi et al., 2009; Li et al., 2010b), in response to light (Yabuta et al., 2007; Yoshimura et al., 2014), ethylene, wounding, cold and post-anoxic conditions (Ioannidi et al., 2009), it seems that this step is not the rate-limiting factor in AsA biosynthesis (Conklin et al., 2006; Torabinejad et al., 2009; Mellidou et al., 2012b; Li et al., 2017). Regarding the other genes downstream this step [L-galactose dehydrogenase (GalDH) and L-galactono-1,4-lactone dehydrogenase (GLDH)], none of them were found to exert a significant effect on AsA pool, at least in tomato fruit (Alhagdow et al., 2007; Mellidou et al., 2012b). Recently however, GLDH has been proposed as a key step in the regulation of AsA accumulation in pepper, being probably involved in the transport of AsA among different organs (Rodríguez-Ruiz et al., 2017).
The most extensively studied alternative route of AsA synthesis is the one through D-galacturonic acid (Figure 1), which is used for the synthesis of L-galactonic acid derivatives via D-galacturonate reductase (GalUR) (Agius et al., 2003; Badejo et al., 2012). Therefore,D-galacturonic acid has a dual role, one being as a key component of cell wall pectins, the other being as a substrate for AsA biosynthesis. Several lines of evidence suggest that AsA synthesis via this pathway may occur in certain species such as strawberry (Agius et al., 2003), orange (Xu et al., 2012), apple (Mellidou et al., 2012a), grape (Cruz-Rus et al., 2010), and rose (Li et al., 2017), or at specific developmental stages i.e., ripe tomato fruit (Badejo et al., 2012). Additionally, increasing AsA levels in tomato plants via expressing the strawberry GalUR has led to enhanced resistance to various abiotic stress factors (Lim M. Y. et al., 2016). Apart from UDP-glucose, the glucoronate pathway for the synthesis of AsA can be also derived from myo-inositol (MI). However, published data suggest that this pathway is primarily involved in hexose metabolism, as well as in starch and cell wall pectin biosynthesis, rather than in AsA biosynthesis, in which MI is quite unlikely to serve as an AsA precursor (Endres and Tenhaken, 2009; Mellidou et al., 2012b). Nevertheless, as this alternative pathway is considerably shorter than the L-galactose pathway, it may complement the predominant biosynthetic route particularly in fruit tissues under stress conditions (Cruz-Rus et al., 2011).
Genetic Regulation Through Enhancing Recycling
As an antioxidant, AsA is able to accept electrons from a wide range of free radicals and in this process it undergoes enzymatic regeneration from its oxidized forms, monodehydroascorbate (MDHA) and dehydroascorbate (DHA). Thereafter, these oxidized forms of AsA can be regenerated by the so-called ascorbate-glutathione (GSH) cycle, so that GSH and the activities of GSH reductase (GR), dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDHAR) preserve AsA homeostasis (Foyer and Noctor, 2011). Overall, the current consensus is that increased AsA levels through enhanced recycling could provide a greater direct protection against free radicals than through increased biosynthesis.
The role of MDHAR in governing AsA pool size has been clearly demonstrated in tomatoes using either QTL mapping and introgression lines (Stevens et al., 2007, 2008; Sauvage et al., 2014), or through assessing expression and activity profiles throughout ripening (Mellidou et al., 2012b). Furthermore, MDHAR expression has been correlated with AsA accumulation in blueberry (Liu et al., 2015). Overexpression of MDHAR had no particular effect on AsA pool in tobacco (Yin et al., 2010), and tomato fruit (Haroldsen et al., 2011), or even exerts a negative effect over the AsA pool (Gest et al., 2013). The unexpected effect of this MDHAR on AsA levels cannot be justified by alterations in the expression of biosynthetic genes, or the activity of recycling enzymes. The authors hypothesized that changes in MDHAR activity in the transgenic lines may trigger a signal that stimulates changes in AsA content and the redox state through an unidentified mechanism of interactions/compensations (Gest et al., 2013). Suppression of MDHAR has led to slight diminished build-up of AsA degradation product in tomatoes, indicating that controlling the recycling rate via reducing MDHAR activity can be an efficient solution toward enhancing the protection of AsA pool (Truffault et al., 2017). Additionally, reduced MDHAR activity via RNAi strategies decreased tolerance to cold storage and affected AsA levels to some extent in tomatoes (El Airaj et al., 2013). To the contrary, overexpression of a chloroplastic MDHAR in tomato led to enhanced AsA concentration (Li et al., 2010a), highlighting the different function that the organelle-specific isoforms exert over the AsA pool.
On the other hand, QTL studies in apple fruit revealed the involvement of DHAR in regulating the redox state of the AsA pool; further, increased flesh DHA concentrations were associated with susceptibility to postharvest disorders such as flesh browning (Mellidou et al., 2012a). Transcriptomic studies uncovered that prolonged postharvest storage downregulated DHAR expression resulting in the irreversibly oxidation of AsA and thus enabling browning to occur (Mellidou et al., 2014). Furthermore, DHAR expression has been correlated with AsA accumulation in chestnut rose (Huang et al., 2014), and in blueberry (Liu et al., 2015). Overexpression of DHAR has been evaluated in several species, and resulted in a remarkable increase of AsA pool in Arabidopsis leaves (Wang et al., 2010), in maize kernels and leaves (Chen et al., 2003; Naqvi et al., 2009), in rice (Kim et al., 2013), as well as in tobacco leaves (Chen et al., 2003; Eltayeb et al., 2006), but not in potato leaves and tubers (Qin et al., 2010). Despite the fact that the initial attempts to enhance AsA recycling yielded no astonishing results, yet the potential value of increasing the efficiency of AsA regeneration should be further studied, mainly because a number of QTLs were linked to DHAR and MDHAR.
Genetic Regulation Through Hormones and Other Proteins/Factors
Recent studies implicate the involvement of hormone interactions in regulating AsA accumulation, a notion previously underestimated. AsA is known to be involved in the synthesis of ethylene as a co-factor of 1-aminocyclopropane-1-carboxylate (ACC) oxidase to convert ACC to ethylene, having thus major roles in fruit development and ripening. In this regard, ACC oxidase is negatively correlated with AsA content in tomatoes (Lima-Silva et al., 2012). Apart from ethylene synthesis, ethylene signaling seems to be involved in AsA accumulation. The increased availability of intermediates for the main biosynthetic pathway due to cell wall pectin degradation triggered by ethylene can increase the flux toward AsA synthesis (Figure 1). Furthermore, the tomato mutants Never-ripe that fail to ripe normally, have high AsA content (Osorio et al., 2012), suggesting a possible negative link between AsA accumulation and ethylene perception that needs to be further explored. Several other genes involved in hormone homeostasis and signaling have been proposed to significantly correlate with AsA levels either positively including the protein phosphatase 2C, which is a negative regulator of abscisic acid (ABA) responses, and gibberellin oxidases, or negatively such as a green ripe-like and a brasinoesteroid-regulated xyloglucan endo-transglycosylase (Lima-Silva et al., 2012). Similarly, co-expression of Stylosanthes guianensis (stylo) 9-cis-epoxycarotenoid dioxygenase and of yeast D-arabinono-1,4-lactone oxidase resulted in increased AsA levels and elevated tolerance to both drought and chilling stress (Bao et al., 2016).
Several TFs have also been proposed to govern AsA levels in plants exposed to oxidative stress or during plant growth (Figure 2). Some of them may exert a positive effect over the AsA pool size through enhancing transcription of the biosynthetic genes such as the ethylene response factor ERF98 (Zhang et al., 2012) in Arabidopsis, or the HD-ZIP I (Hu et al., 2016) and the auxin/indolo acetic acid (Aux/IAA) TF in tomato (Lima-Silva et al., 2012). Others may exert a negative impact on AsA concentrations such as the ERF33 in tomato (Lima-Silva et al., 2012), or may regulate AsA-dependent growth such as the ABSCISIC ACID-INSENSITIVE-4 TF (ABI4; Kerchev et al., 2011) in Arabidopsis. Similar results have been recorded in ripening tomato fruit, with several TFs including MYB, NAC and ZIF, modulating transcript levels of AsA biosynthetic genes, and correlating with the accumulation of AsA (Ye et al., 2015). Nevertheless, overexpression or reducing the expression of ERF98 and HD-ZIP I showed a moderate effect on the AsA concentration (Bulley and Laing, 2016), questioning their significance in governing AsA pool. In addition, some TFs significantly correlated with DHA accumulation, rather than total AsA abundance (Ye et al., 2015), indicating the potential linkage between TFs and redox homeostasis.
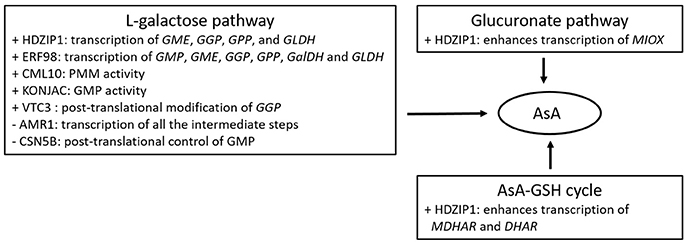
Figure 2. Summary of proteins/factors affecting either positively (+), or negatively (−) the AsA metabolic pathways.
There are also factors affecting enzyme or TF concentrations, such as VTC3 (Conklin et al., 2013), a component of the photomorphogenic COP9 signalosome (CSN5B; Wang J. et al., 2013), and AMR1 (AsA mannose pathway regulator 1; Zhang et al., 2009), all identified in Arabidopsis (Figure 2). VTC3, the last AsA-deficient Arabidopsis mutant loci with unknown function, has been predicted to encode a novel highly conserved dual function protein kinase/protein phosphatase 2C (Conklin et al., 2013). With two signal transduction domains, VTC3 may be involved in signal transduction regulating AsA levels in response to adverse environments. Furthermore, as its transcripts are constantly expressed across a wide range of conditions, its control over AsA pool may occur at the posttranslational level. Bulley and Laing (2016) proposed that VTC3 may be implicated in regulating the noncanonical highly conserved uORF of GGP. On the other hand, CSN5B and AMR1 exert a negative role over AsA levels in that a decrease in their transcript levels enhance AsA accumulation. Interestingly, a decrease in the expression of AMR1 led to a 2-fold increase of AsA concentrations, as well as a boost in transcript levels of GGP, GME and GMP (Zhang et al., 2009). On the other hand, CSN5B was also found to interact with GMP affecting the light-dark control of AsA biosynthesis, indicating that CSN5B may be a potential posttranslational regulator of AsA synthesis (Wang J. et al., 2013; Bulley and Laing, 2016). A point mutation of VTC1 impairs the interaction with CSN5B and results in increased AsA biosynthesis and Arabidopsis seedling growth (Li et al., 2016). Other factors including nucleotide sugar pyrophosphorylase-like proteins (KONJAC1 and 2; Sawake et al., 2015), and a calmodulin-like protein (CML10; Cho et al., 2016), have been found to stimulate enzyme activity of AsA-related genes, revealing novel mechanisms of how the AsA pool is regulated. Specifically, KONJAC1 and 2 can stimulate GMP activity, while CML10 the activity of PMM resulting in increased cellular levels of mannose-1-phospate and consequently the subsequent reactions in the AsA biosynthetic pathway. Reducing gene expression of KONJACs and CML10 led to a 0.4 and 0.5–0.75-fold change in AsA concentrations, respectively (Bulley and Laing, 2016). As all previous reports have been conducted on model plant species, it remains an open question whether these factors operate similarly in horticultural crops.
Conclusion and Perspective
Emerging evidence provides important clues on the mechanism by which GGP, the key AsA biosynthetic gene, regulates the AsA pool size in several species, either through variations in GGP transcript levels or through feedback control at the translational level. Recent advances clearly suggest that inducing or inhibiting GGP translation via a highly conserved uORF may serve as a prominent mechanism to control the AsA accumulation rapidly under unfavorable environmental conditions. On the contrary, plethora of studies reinforce the hypothesis that induction of the other genes of the L-galactose pathway may be important in certain species, tissues, or developmental stages and may be responsible for the tissue acclimation under slowly changing conditions, or at certain environmental/developmental conditions. Further, it seems that the AsA pool can be also regulated via other metabolic pathways than biosynthesis and recycling that could affect either substrate availability, or control the expression levels of AsA-related genes at either the transcriptional or posttranslational level. However, recent advances on genetic engineering using either AsA structural genes or TFs and other factors clearly suggest that improving AsA concentrations beyond certain thresholds is harder than initially believed. Therefore, efforts should be directed toward understanding whether structural genes and TFs act individually or collectively to govern AsA accumulation, as well as what is the effect of environment on these systems in horticultural crops rather than model species. It also remains an open question why wild accessions tend to contain higher AsA levels than modern cultivars (up to 5-fold in tomato), or what is the source of variation within species (up to 10-fold in kiwifruit), and which genes/factors govern these mechanisms.
Author Contributions
IM organized and drafted this manuscript and AKK contributed to the editing of the manuscript. All authors read and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Work cited in this work was supported by grants to AKK (EU-SOL-FOOD-CT-2006-016214, GR-NUTRITOM/ 11Syn_3_480, GR-PYTHAGORAS – EPEAEK II, GSRT-GR-UK joint program in Agricultural Biotechnology, and COST Action FA1106 “QualityFruit”).
References
Agius, F., González-Lamothe, R., Caballero, J. L., Muñoz-Blanco, J., Botella, M. A., and Valpuesta, V. (2003). Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 21, 177–181. doi: 10.1038/nbt777
Alhagdow, M., Mounet, F., Gilbert, L., Nunes-Nesi, A., Garcia, V., Just, D., et al. (2007). Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in Tomato. Plant Physiol. 145, 1408–1422. doi: 10.1104/pp.107.106500
Alós, E., Rodrigo, M. J., and Zacarías, L. (2013). Transcriptomic analysis of genes involved in the biosynthesis, recycling and degradation of L-ascorbic acid in pepper fruits (Capsicum annuum L.). Plant Sci. 207, 2–11. doi: 10.1016/j.plantsci.2013.02.007
Badejo, A. A., Tanaka, N., and Esaka, M. (2008). Analysis of GDP-D-mannose pyrophosphorylase gene promoter from acerola (Malpighia glabra) and increase in ascorbate content of transgenic tobacco expressing the acerola gene. Plant Cell Physiol. 49, 126–132. doi: 10.1093/pcp/pcm164
Badejo, A. A., Wada, K., Gao, Y., Maruta, T., Sawa, Y., Shigeoka, S., et al. (2012). Translocation and the alternative D-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the D-mannose/L-galactose pathway. J. Exp. Bot. 63, 229–239. doi: 10.1093/jxb/err275
Bao, G., Zhuo, C., Qian, C., Xiao, T., Guo, Z., and Lu, S. (2016). Co-expression of NCED and ALO improves vitamin C level and tolerance to drought and chilling in transgenic tobacco and stylo plants. Plant Biotechnol. J. 14, 206–214. doi: 10.1111/pbi.12374
Bulley, S., and Laing, W. (2016). The regulation of ascorbate biosynthesis. Curr. Opin. Plant Biol. 33, 15–22. doi: 10.1016/j.pbi.2016.04.010
Bulley, S. M., Rassam, M., Hoser, D., Otto, W., Schuenemann, N., Wright, M., et al. (2009). Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J. Exp. Bot. 60, 765–778. doi: 10.1093/jxb/ern327
Bulley, S., Wright, M., Rommens, C., Yan, H., Rassam, M., Lin-Wang, K., et al. (2012). Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnol. J. 10, 390–397. doi: 10.1111/j.1467-7652.2011.00668.x
Chen, Z., Young, T. E., Ling, J., Chang, S.-C., and Gallie, D. R. (2003). Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. U.S.A. 100, 3525–3530. doi: 10.1073/pnas.0635176100
Cho, K.-M., Nguyen, H. T. K., Kim, S. Y., Shin, J. S., Cho, D. H., Hong, S. B., et al. (2016). CML10, a variant of calmodulin, modulates ascorbic acid synthesis. New Phytol. 209, 664–678. doi: 10.1111/nph.13612
Conklin, P. L., DePaolo, D., Wintle, B., Schatz, C., and Buckenmeyer, G. (2013). Identification of Arabidopsis VTC3 as a putative and unique dual function protein kinase::protein phosphatase involved in the regulation of the ascorbic acid pool in plants. J. Exp. Bot. 64, 2793–2804. doi: 10.1093/jxb/ert140
Conklin, P. L., Gatzek, S., Wheeler, G. L., Dowdle, J., Raymond, M. J., Rolinski, S., et al. (2006). Arabidopsis thaliana VTC4 encodes L-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. J. Biol. Chem. 281, 15662–15670. doi: 10.1074/jbc.M601409200
Conklin, P. L., Saracco, S. A., Norris, S. R., and Last, R. L. (2000). Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154, 847–856.
Cruz-Rus, E., Amaya, I., Sanchez-Sevilla, J. F., Botella, M. A., and Valpuesta, V. (2011). Regulation of L-ascorbic acid content in strawberry fruits. J. Exp. Bot. 62, 4191–4201. doi: 10.1093/jxb/err122
Cruz-Rus, E., Botella, M. A., Valpuesta, V., and Gomez-Jimenez, M. C. (2010). Analysis of genes involved in l-ascorbic acid biosynthesis during growth and ripening of grape berries. J. Plant Physiol. 167, 739–748. doi: 10.1016/j.jplph.2009.12.017
Davey, M. W., Kenis, K., and Keulemans, J. (2006). Genetic control of fruit vitamin C contents. Plant Physiol. 142, 343–351. doi: 10.1104/pp.106.083279
Dowdle, J., Ishikawa, T., Gatzek, S., Rolinski, S., and Smirnoff, N. (2007). Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 52, 673–689. doi: 10.1111/j.1365-313X.2007.03266.x
El Airaj, H., Gest, N., Truffault, V., Garchery, C., Riqueau, G., Gouble, B., et al. (2013). Decreased monodehydroascorbate reductase activity reduces tolerance to cold storage in tomato and affects fruit antioxidant levels. Postharvest Biol. Technol. 86, 502–510. doi: 10.1016/j.postharvbio.2013.07.035
Eltayeb, A. E., Kawano, N., Badawi, G. H., Kaminaka, H., Sanekata, T., Morishima, I., et al. (2006). Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiol. Plant. 127, 57–65. doi: 10.1111/j.1399-3054.2006.00624.x
Endres, S., and Tenhaken, R. (2009). Myoinositol oxygenase controls the level of myoinositol in Arabidopsis, but does not increase ascorbic acid. Plant Physiol. 149, 1042–1049. doi: 10.1104/pp.108.130948
Fotopoulos, V., De Tullio, M. C., Barnes, J., and Kanellis, A. K. (2008). Altered stomatal dynamics in ascorbate oxidase over-expressing tobacco plants suggest a role for dehydroascorbate signalling. J. Exp. Bot. 59, 729–737. doi: 10.1093/jxb/erm359
Fotopoulos, V., and Kanellis, A. K. (2013). Plant physiology and biochemistry Altered apoplastic ascorbate redox state in tobacco plants via ascorbate oxidase overexpression results in delayed dark-induced senescence in detached leaves. Plant Physiol. Biochem. 73, 154–160. doi: 10.1016/j.plaphy.2013.09.002
Fotopoulos, V., Sanmartin, M., and Kanellis, A. K. (2006). Effect of ascorbate oxidase over-expression on ascorbate recycling gene expression in response to agents imposing oxidative stress. J. Exp. Bot. 57, 3933–3943. doi: 10.1093/jxb/erl147
Foyer, C. H., and Noctor, G. (2011). Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 155, 2–18. doi: 10.1104/pp.110.167569
Gest, N., Gautier, H., and Stevens, R. (2013). Ascorbate as seen through plant evolution: the rise of a successful molecule? J. Exp. Bot. 64, 33–53. doi: 10.1093/jxb/ers297
Gilbert, L., Alhagdow, M., Nunes-Nesi, A., Quemener, B., Guillon, F., Bouchet, B., et al. (2009). GDP-d-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J. 60, 499–508. doi: 10.1111/j.1365-313X.2009.03972.x
Haroldsen, V. M., Chi-Ham, C. L., Kulkarni, S., Lorence, A., and Bennett, A. B. (2011). Constitutively expressed DHAR and MDHAR influence fruit, but not foliar ascorbate levels in tomato. Plant Physiol. Biochem. 49, 1244–1249. doi: 10.1016/j.plaphy.2011.08.003
Hu, T., Ye, J., Tao, P., Li, H., Zhang, J., Zhang, Y., et al. (2016). The tomato HD-Zip i transcription factor SlHZ24 modulates ascorbate accumulation through positive regulation of the d -mannose/ l -galactose pathway. Plant J. 85, 16–29. doi: 10.1111/tpj.13085
Huang, M., Xu, Q., and Deng, X.-X. (2014). l-Ascorbic acid metabolism during fruit development in an ascorbate-rich fruit crop chestnut rose (Rosa roxburghii Tratt). J. Plant Physiol. 171, 1205–1216. doi: 10.1016/j.jplph.2014.03.010
Imai, T., Ban, Y., Terakami, S., Yamamoto, T., and Moriguchi, T. (2009). L-Ascorbate biosynthesis in peach: cloning of six L-galactose pathway-related genes and their expression during peach fruit development. Physiol. Plant. 136, 139–149. doi: 10.1111/j.1399-3054.2009.01213.x
Ioannidi, E., Kalamaki, M. S., Engineer, C., Pateraki, I., Alexandrou, D., Mellidou, I., et al. (2009). Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J. Exp. Bot. 60, 663–678. doi: 10.1093/jxb/ern322
Jain, A. K., and Nessler, C. L. (2000). Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Mol. Breed. 6, 73–78. doi: 10.1023/A:1009680818138
Kerchev, P. I., Pellny, T. K., Vivancos, P. D., Kiddle, G., Hedden, P., Driscoll, S., et al. (2011). The transcription factor ABI4 Is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell 23, 3319–3334. doi: 10.1105/tpc.111.090100
Kim, Y.-S., Kim, I.-S., Bae, M.-J., Choe, Y.-H., Kim, Y.-H., Park, H.-M., et al. (2013). Homologous expression of cytosolic dehydroascorbate reductase increases grain yield and biomass under paddy field conditions in transgenic rice (Oryza sativa L. japonica). Planta 237, 1613–1625. doi: 10.1007/s00425-013-1862-8
Laing, W. A., Martinez-Sanchez, M., Wright, M. A., Bulley, S. M., Brewster, D., Dare, A. P., et al. (2015). An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in Arabidopsis. Plant Cell 27, 772–786. doi: 10.1105/tpc.114.133777
Laing, W. A., Wright, M. A., Cooney, J., and Bulley, S. M. (2007). The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc. Natl. Acad. Sci. U.S.A. 104, 9534–9539. doi: 10.1073/pnas.0701625104
Li, F., Wu, Q.-Y., Sun, Y.-L., Wang, L.-Y., Yang, X.-H., and Meng, Q.-W. (2010a). Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol. Plant. 139, 421–434. doi: 10.1111/j.1399-3054.2010.01369.x
Li, J., Li, M., Liang, D., Ma, F., and Lei, Y. (2014). Comparison of expression pattern, genomic structure, and promoter analysis of the gene encoding GDP-l-galactose phosphorylase from two Actinidia species. Sci. Hortic. 169, 206–213. doi: 10.1016/j.scienta.2014.02.024
Li, L., Lu, M., and An, H. (2017). Expression profiles of the genes involved in l-ascorbic acid biosynthesis and recycling in Rosa roxburghii leaves of various ages. Acta Physiol. Plant. 39, 44–53. doi: 10.1007/s11738-016-2346-9
Li, M., Ma, F., Guo, C., and Liu, J. (2010b). Ascorbic acid formation and profiling of genes expressed in its synthesis and recycling in apple leaves of different ages. Plant Physiol. Biochem. 48, 216–224. doi: 10.1016/j.plaphy.2010.01.015
Li, M., Ma, F., Liang, D., Li, J., and Wang, Y. (2010c). Ascorbate biosynthesis during early fruit development is the main reason for its accumulation in kiwi. PLoS ONE 5:e14281. doi: 10.1371/journal.pone.0014281
Li, S., Wang, J., Yu, Y., Wang, F., Dong, J., and Huang, R. (2016). D27E mutation of VTC1 impairs the interaction with CSN5B and enhances ascorbic acid biosynthesis and seedling growth in Arabidopsis. Plant Mol. Biol. 92, 473–482. doi: 10.1007/s11103-016-0525-0
Lim, B., Smirnoff, N., Cobbett, C. S., and Golz, J. F. (2016). Ascorbate-deficient vtc2 mutants in Arabidopsis do not exhibit decreased growth. Front. Plant Sci. 7:1025. doi: 10.3389/fpls.2016.01025
Lim, M. Y., Jeong, B. R., Jung, M., and Harn, C. H. (2016). Transgenic tomato plants expressing strawberry d-galacturonic acid reductase gene display enhanced tolerance to abiotic stresses. Plant Biotechnol. Rep. 10, 105–116. doi: 10.1007/s11816-016-0392-9
Lim, M. Y., Pulla, R. K., Park, J. M., Harn, C. H., and Jeong, B. R. (2012). Over-expression of l-gulono-γ-lactone oxidase (GLOase) gene leads to ascorbate accumulation with enhanced abiotic stress tolerance in tomato. Vitr. Cell. Dev. Biol. Plant 48, 453–461. doi: 10.1007/s11627-012-9461-0
Lima-Silva, V., Rosado, A., Amorim-Silva, V., Muñoz-Mérida, A., Pons, C., Bombarely, A., et al. (2012). Genetic and genome-wide transcriptomic analyses identify co-regulation of oxidative response and hormone transcript abundance with vitamin C content in tomato fruit. BMC Genomics 13:187. doi: 10.1186/1471-2164-13-187
Liu, F., Wang, L., Gu, L., Zhao, W., Su, H., and Cheng, X. (2015). Higher transcription levels in ascorbic acid biosynthetic and recycling genes were associated with higher ascorbic acid accumulation in blueberry. Food Chem. 188, 399–405. doi: 10.1016/j.foodchem.2015.05.036
Maruta, T., Yonemitsu, M., Yabuta, Y., Tamoi, M., Ishikawa, T., and Shigeoka, S. (2008). Arabidopsis phosphomannose isomerase 1, but not phosphomannose isomerase 2, is essential for ascorbic acid biosynthesis. J. Biol. Chem. 283, 28842–28851. doi: 10.1074/jbc.M805538200
Massot, C., Stevens, R., Génard, M., Longuenesse, J.-J., and Gautier, H. (2012). Light affects ascorbate content and ascorbate-related gene expression in tomato leaves more than in fruits. Planta 235, 153–163. doi: 10.1007/s00425-011-1493-x
Mellidou, I., Buts, K., Hatoum, D., Ho, Q. T., Johnston, J. W., Watkins, C. B., et al. (2014). Transcriptomic events associated with internal browning of apple during postharvest storage. BMC Plant Biol. 14:328. doi: 10.1186/s12870-014-0328-x
Mellidou, I., Chagné, D., Laing, W. A., Keulemans, J., and Davey, M. W. (2012a). Allelic variation in paralogs of GDP-L-galactose phosphorylase is a major determinant of vitamin C concentrations in apple Fruit. Plant Physiol. 160, 1613–1629. doi: 10.1104/pp.112.203786
Mellidou, I., Keulemans, J., Kanellis, A. K., and Davey, M. W. (2012b). Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol. 12:239. doi: 10.1186/1471-2229-12-239
Mounet-Gilbert, L., Dumont, M., Ferrand, C., Bournonville, C., Monier, A., Jorly, J., et al. (2016). Two tomato GDP-D-mannose epimerase isoforms involved in ascorbate biosynthesis play specific roles in cell wall biosynthesis and development. J. Exp. Bot. 67, 4767–4777. doi: 10.1093/jxb/erw260
Naqvi, S., Zhu, C., Farre, G., Ramessar, K., Bassie, L., Breitenbach, J., et al. (2009). Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc. Natl. Acad. Sci. U.S.A. 106, 7762–7767. doi: 10.1073/pnas.0901412106
Osorio, S., Alba, R., Nikoloski, Z., Kochevenko, A., Fernie, A. R., and Giovannoni, J. J. (2012). Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiol. 159, 1713–1729. doi: 10.1104/pp.112.199711
Pateraki, I., Sanmartin, M., Kalamaki, M. S., Gerasopoulos, D., and Kanellis, A. K. (2004). Molecular characterization and expression studies during melon fruit development and ripening of L-galactono-1,4-lactone dehydrogenase. J. Exp. Bot. 55, 1623–1633. doi: 10.1093/jxb/erh186
Qian, W., Yu, C., Qin, H., Liu, X., Zhang, A., Johansen, I. E., et al. (2007). Molecular and functional analysis of phosphomannomutase (PMM) from higher plants and genetic evidence for the involvement of PMM in ascorbic acid biosynthesis in Arabidopsis and Nicotiana benthamiana. Plant J. 49, 399–413. doi: 10.1111/j.1365-313X.2006.02967.x
Qin, A., Shi, Q., and Yu, X. (2010). Ascorbic acid contents in transgenic potato plants overexpressing two dehydroascorbate reductase genes. Mol. Biol. Rep. 38, 1557–1566. doi: 10.1007/s11033-010-0264-2
Rodríguez-Ruiz, M., Mateos, R. M., Codesido, V., Corpas, F. J., and Palma, J. M. (2017). Characterization of the galactono-1,4-lactone dehydrogenase from pepper fruits and its modulation in the ascorbate biosynthesis. Role of nitric oxide. Redox Biol. 12, 171–181. doi: 10.1016/j.redox.2017.02.009
Sanmartin, M., Drogoudi, P. A., Lyons, T., Pateraki, I., Barnes, J., and Kanellis, A. K. (2003). Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta 216, 918–928. doi: 10.1007/s00425-002-0944-9
Sanmartin, M., Pateraki, I., Chatzopoulou, F., and Kanellis, A. K. (2007). Differential expression of the ascorbate oxidase multigene family during fruit development and in response to stress. Planta 225, 873–885. doi: 10.1007/s00425-006-0399-5
Sauvage, C., Segura, V., Bauchet, G., Stevens, R., Do, P. T., Nikoloski, Z., et al. (2014). Genome-wide association in tomato reveals 44 candidate loci for fruit metabolic traits. Plant Physiol. 165, 1120–1132. doi: 10.1104/pp.114.241521
Sawake, S., Tajima, N., Mortimer, J. C., Lao, J., Ishikawa, T., Yu, X., et al. (2015). KONJAC1 and 2 are key factors for GDP-mannose generation and affect l-Ascorbic acid and glucomannan biosynthesis in arabidopsis. Plant Cell 27, 3397–3409. doi: 10.1105/tpc.15.00379
Smirnoff, N. (2000). Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 3, 229–35. doi: 10.1016/S1369-5266(00)00069-8
Stevens, R., Buret, M., Duffé, P., Garchery, C., Baldet, P., Rothan, C., et al. (2007). Candidate genes and quantitative trait loci affecting fruit ascorbic acid content in three tomato populations. Plant Physiol. 143, 1943–1953. doi: 10.1104/pp.106.091413
Stevens, R., Page, D., Gouble, B., Garchery, C., Zamir, D., and Causse, M. (2008). Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ. 31, 1086–1096. doi: 10.1111/j.1365-3040.2008.01824.x
Torabinejad, J., Donahue, J. L., Gunesekera, B. N., Allen-Daniels, M. J., and Gillaspy, G. E. (2009). VTC4 is a bifunctional enzyme that affects myoinositol and ascorbate biosynthesis in plants. Plant Physiol. 150, 951–961. doi: 10.1104/pp.108.135129
Troesch, B., Hoeft, B., McBurney, M., Eggersdorfer, M., and Weber, P. (2012). Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 108, 692–698. doi: 10.1017/S0007114512001808
Truffault, V., Fry, S. C., Stevens, R. G., and Gautier, H. (2017). Ascorbate degradation in tomato leads to accumulation of oxalate, threonate and oxalyl threonate. Plant J. 89, 996–1008. doi: 10.1111/tpj.13439
Wang, J., Yu, Y., Zhang, Z., Quan, R., Zhang, H., Ma, L., et al. (2013). Arabidopsis CSN5B interacts with VTC1 and modulates Ascorbic acid synthesis. Plant Cell 25, 1–13. doi: 10.1105/tpc.112.106880
Wang, L.-Y., Li, D., Deng, Y.-S., Lv, W., and Meng, Q.-W. (2013). Antisense-mediated depletion of tomato GDP-l-galactose phosphorylase increases susceptibility to chilling stress. J. Plant Physiol. 170, 303–314. doi: 10.1016/j.jplph.2012.10.015
Wang, L.-Y., Meng, X., Yang, D., Ma, N., Wang, G., and Meng, Q. (2014). Overexpression of tomato GDP-l-galactose phosphorylase gene in tobacco improves tolerance to chilling stress. Plant Cell Rep. 33, 1441–1451. doi: 10.1007/s00299-014-1627-2
Wang, Z., Xiao, Y., Chen, W., Tang, K., and Zhang, L. (2010). Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J. Integr. Plant Biol. 52, 400–409. doi: 10.1111/j.1744-7909.2010.00921.x
Wheeler, G., Ishikawa, T., Pornsaksit, V., Smirnoff, N., Abascal, F., Zardoya, R., et al. (2015). Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. Elife 4, 2104–2105. doi: 10.7554/eLife.06369
Wheeler, G. L., Jones, M. A., and Smirnoff, N. (1998). The biosynthetic pathway of vitamin C in higher plants. Nature 393, 365–369. doi: 10.1038/30728
Wolucka, B. A., and Van Montagu, M. (2003). GDP-mannose 3',5'-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 278, 47483–47490. doi: 10.1074/jbc.M309135200
Xu, Q., Chen, L.-L., Ruan, X., Chen, D., Zhu, A., Chen, C., et al. (2012). The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 45, 59–66. doi: 10.1038/ng.2472
Yabuta, Y., Mieda, T., Rapolu, M., Nakamura, A., Motoki, T., Maruta, T., et al. (2007). Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 58, 2661–2671. doi: 10.1093/jxb/erm124
Ye, J., Hu, T., Yang, C., Li, H., Yang, M., Ijaz, R., et al. (2015). Transcriptome profiling of tomato fruit development reveals transcription factors associated with ascorbic acid, carotenoid and flavonoid biosynthesis. PLoS ONE 10:e0130885. doi: 10.1371/journal.pone.0130885
Yin, L., Wang, S., Eltayeb, A. E., Uddin, M. I., Yamamoto, Y., Tsuji, W., et al. (2010). Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231, 609–621. doi: 10.1007/s00425-009-1075-3
Yoshimura, K., Nakane, T., Kume, S., Shiomi, Y., Maruta, T., Ishikawa, T., et al. (2014). Transient expression analysis revealed the importance of VTC2 expression level in light/dark regulation of ascorbate biosynthesis in Arabidopsis. Biosci. Biotechnol. Biochem. 78, 60–66. doi: 10.1080/09168451.2014.877831
Zhang, C., Liu, J., Zhang, Y., Cai, X., Gong, P., Zhang, J., et al. (2010). Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 30, 389–398. doi: 10.1007/s00299-010-0939-0
Zhang, W., Lorence, A., Gruszewski, H. A., Chevone, B. I., and Nessler, C. L. (2009). AMR1, an Arabidopsis gene that coordinately and negatively regulates the mannose/l-galactose ascorbic acid biosynthetic pathway. Plant Physiol. 150, 942–950. doi: 10.1104/pp.109.138453
Keywords: ascorbate biosynthesis, GGP, ascorbate recycling, translation, transcription, vitamin C
Citation: Mellidou I and Kanellis AK (2017) Genetic Control of Ascorbic Acid Biosynthesis and Recycling in Horticultural Crops. Front. Chem. 5:50. doi: 10.3389/fchem.2017.00050
Received: 27 March 2017; Accepted: 27 June 2017;
Published: 11 July 2017.
Edited by:
Dominique Van Der Straeten, Ghent University, BelgiumReviewed by:
Ravinder K. Goyal, Agriculture and Agri-Food Canada, CanadaPanagiotis Kalaitzis, Mediterranean Agronomic Institute of Chania, Greece
Copyright © 2017 Mellidou and Kanellis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angelos K. Kanellis, kanellis@pharm.auth.gr
 Ifigeneia Mellidou
Ifigeneia Mellidou Angelos K. Kanellis
Angelos K. Kanellis