Abstract
Near infrared spectroscopy (NIRS) has been used to measure concentration changes of cerebral hemoglobin and cytochrome in neonates, children, and adults, to study cerebral oxygenation and hemodynamics. To derive quantitative concentration changes from measurements of light attenuation, the optical path length must be known. This is obtained by multiplying the source/detector separation by a laboratory measured differential path length factor (DPF) which accounts for the increased distance traveled by light due to scattering. DPF has been measured by time of flight techniques on small populations of adults and postmortem infants. The values for adults are greater than those for newborns, and it is not clear how to interpolate the present data for studies on children. Recent developments in instrumentation using phase resolved spectroscopy techniques have produced a bedside unit which can measure optical path length on any subject. We have developed an intensity modulated optical spectrometer which measures path length at four wavelengths. Two hundred and eighty three subjects from 1 d of age to 50 y were studied. Measurements were made at a fixed frequency of 200 MHz and a source detector separation of 4.5 cm. Results suggest a slowly varying age dependence of DPF, following the relation DPF690 = 5.38 + 0.049A0.877, DPF744 = 5.11 + 0.106A0.723, DPF807 = 4.99 + 0.067A0.814, and DPF832 = 4.67 + 0.062A0.819, where DPF690 is the DPF measured at 690 nm and A is age is expressed in years from full term. There was a wide scatter of values, however, implying that ideally DPF should be measured at the time of each study.
Similar content being viewed by others
Main
NIRS has been shown to be a viable noninvasive technique for monitoring cerebral oxygenation and hemodynamics in the newborn infant at the bedside(1–4) and in adults(5–8). Results suggest possibilities of observing brain oxygenation in the fetus during delivery(9, 10) and in children undergoing cardiac surgery(11–13). More recently, by the application of small physiologic maneuvers, absolute quantitation of important hemodynamic variables such as blood flow, volume, and average tissue hemoglobin saturation have been achieved(7, 13–15). Current commercial instruments measure attenuation changes of a number of wavelengths of near infrared light transmitted through the tissue. Using the known absorption coefficient of each chromophore at the wavelengths used, the source/detector separation and a DPF, a modified Beer Lambert law is applied to solve for concentration change of each chromophore(16). The source/detector separation and the DPF when multiplied together yield the mean propagation distance of the light detected. The DPF is around 3-6 due to the highly scattering nature of light in tissue, but has been found to be approximately constant with optode spacing for tissue type if the separation is over 2.5 cm(17–20). Quantitation of NIRS data relies on an accurate evaluation of DPF.
The DPF currently in use for neonates and adults were determined by measuring the TOF for a picosecond pulse of light to travel through tissue(21). Unfortunately the size and expense of typical laboratory equipment needed for these measurements precludes the possibility of measurements at the bedside or on normal newborns and children, although recently Benaron et al.(22) described a bedside TOF system. Results with TOF systems have usually relied on small populations (typically <10) with values available only for adult volunteers and postmortem neonates. The resulting data for DPF, 5.93 ± 7.08% at 761 nm and 6.32 ± 7.28% at 800 nm for the adult head(20, 21) with 3.85 ± 14.8% and 4.39± 6.38% both at 760 nm for neonates(20, 23), show both a large SD and an increase from neonates to adults. Cooperet al.(24) have recently reported similar values for optical path lengths in a group of neonates measured by second derivative NIRS of water. Table 1 summarizes published values of DPF measured by a variety of methods in groups of adults and neonates. Although it is clear from these data that DPF does increase with age, the age dependence over the pediatric and adolescent period has not been established, which produces a dilemma if accurate quantitative results are required for a child or an adolescent(13).
Recently an alternative method of measuring path length using PRS has been developed(25, 26). Benaron et al.(27) used a simple single-frequency (200 MHz), two-wavelength PRS instrument to measure optical path length in a group of 34 infants aged 1 d to 3 y, but the small optode spacing and the wide range of pathologies make interpretation of the data difficult. PRS uses radiofrequency intensity modulated continuous light sources. Detection is via a photomultiplier tube, and the DC and AC components describe the attenuation and phase shift of the light through the tissue, respectively. Phase shift can be related to mean path length with a knowledge of the modulation frequency and the tissue refractive index(28). We have developed a broadband radiofrequency-modulated PRS instrument using four wavelengths(690, 744, 807, and 832 nm) which can measure phase shifts through >4 cm of brain tissue in less than 1 s. [For this study the modulation frequency was set at 200 MHz which has been shown theoretically to be a frequency at which phase shift and true mean optical path length are equal(29).] As the equipment is portable and potentially compact, real time path length measurement at the bedside is now possible. In addition the noninvasive procedure and speed of data collection and analysis(5 s) offer the chance to record large populations and any age group. The aim of this study was to determine the age dependence of DPF between the neonatal and adult groups to provide an estimate of DPF for those NIRS users involved in pediatric studies.
METHODS
Subjects. In total, 283 subjects between the ages of 1 d and 50 y were studied. There were 137 male and 146 female subjects. Due to the spread of values the data were divided into age bands. Those studied within 1 wk of birth (gestational age range 35-42 wk) were grouped in the“neonates” band. Children up to and including 15 y old were grouped in 1-y age bands. Above 15 y the sample sizes were smaller, and 5-y age bands were used. Figure 1 shows the distribution of subjects in each group, plotted halfway at the mean value of the age band. The 35 newborn infants and all of the adults aged 20 y and older were studied at University College Hospital, the infants within the Obstetrics hospital, and the adults within the Department of Medical Physics and Bioengineering and the Neonatal Unit (nursing staff volunteers). All remaining children were studied at Great Ormond Street Hospital for Children NHS Trust. In all cases the studies were approved by the relevant hospital committee on the ethics of human research, and informed written consent was obtained from each adult subject or the parents of those aged 16 y and under. All the infants studied at University College Hospital had no known cerebral or cardiovascular abnormalities, and all adult volunteers were healthy. At the Hospital for Sick Children the majority of volunteers were healthy (group A), whereas 44 had a medical condition which was not expected to affect their cerebral path length and were considered as normal for the purposes of this study (group B). Sixteen children had disorders affecting the central nervous system or the skull, which could potentially lead to abnormal DPF values (group C).Table 2 shows the diagnoses of the children in group C. The mean values of DPF for the adult and neonatal subjects have been published(30).
Measurement procedure. An intensity modulated optical spectrophotometer operating at 200 MHz was used(26). To measure absolute optical path length and determine DPF, a reference phase signal (REF) representing zero path length was initially taken at various levels of photomultiplier tube gain, by placing the optodes together separated by a known thickness of attenuating material (used to avoid saturation of the photomultiplier tube). The optodes were two optical fiber delivery systems which rested on the skin surface, one delivering the laser light, whereas the second returned the detected light from the tissue to the photomultiplier tube. For the measurements they were fixed into a black silicon rubber mold with a fixed separation of 4.3 cm. Once these were placed upon the volunteer, the sensitivity of the photomultiplier tube was increased until adequate detection of light at the four wavelengths was achieved. At this point the phase shift was recorded at 0.5-s intervals for 5 s. The phase shift was then averaged, φDATA, and compared with the phase shift recorded for zero path length at the same photomultiplier tube sensitivity,φREF. Absolute path length, d, is given by(29): Equation where c is the speed of light in vacuo, f is the modulation frequency (200 MHz), and n = 1.4, the refractive index of tissue(28). The DPF is simply Equation and is displayed for the four wavelengths, 690, 744, 807, and 832 nm.


The optodes were positioned on the left side of the forehead just below the hairline. For infants and younger children the lateral optode was placed in a temperoparietal position, and the left or right side was used to minimize disturbance to the child.
RESULTS
Subject measurement repeatability. The calculated DPF depends on a comparison between two measurements of phase shift, and therefore depends upon the stability of the instrument and the reference phase shift(φREF). To examine this and the likely physiologic variability of the signal, a series of repeated measurements on a subject were performed(30). The arm of a female volunteer, aged 26 y, was used. The arm was supported in a resting position, palm facing downward, with the optodes under the forearm. The source optode was 13.5 cm from the wrist and the detector optode 4.5 cm closer to the elbow. The arm remained in place to ensure consistent positioning, and the phase shift and absolute path length were recorded for 10 repeated measurements over a period of 5 min using a single reference phase shift. A SD of only 0.55% at 690 nm, 0.59% at 744 nm, 0.61% at 807 nm, and 0.71% at 832 nm on the optical path length was observed.
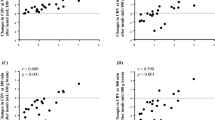
DPF variability with age. Figure 2 shows the variation of DPF with age for the entire age range at a wavelength of 807 nm. There is considerable inhomogeneity of variance, ranging from 0.04 to 1.46. Diffusion theory for light transport in tissue predicts that in a homogeneous medium DPF should vary with the square root of the scattering coefficient, and inversely with the square root of the absorption. Modelling also predicts an increase in DPF with head diameter(32). Assuming that any of these parameters vary linearly with age, one might expect DPF to follow a power law relationship with age. The results of fitting this type of relation to the data at 807 nm is shown in Figure 3. The equation was fitted to the original data points using a least squares fitting routine, and the intercept at age zero was taken to be the mean DPF for the neonatal group. This was felt to be justified given the relatively large number in the group(35) and the small age spread (median gestational age 40.1 wk, range 35.1-42.5 wk). The equation of the curve is: Equation where A = age in years. The equations for the other wavelengths are given in Table 3 together with mean and SD of the DPF values for the neonatal group. The DPF values are not considered for the male and female subjects separately as previous studies have already shown that cerebral DPF does not vary with the sex of the subject(30).

DPF variation with wavelength. A wavelength dependence of DPF has previously been shown on a group of six adults using a laboratory-based TOF system(21). Figure 2 shows the variation of DPF with age at each wavelength. As expected the DPF drops as the wavelength increases. The wavelength dependence is approximately uniform with age.
DPF variation with brain abnormalities. Sixteen of the children studied were considered to have a clinical condition that could affect the cerebral path length. They are listed in Table 2. For illustration only the data at 807 nm are used, and Figure 4 shows a graph of the DPF variation over the relevant age range for the normal head with the 16 DPF values recorded for the group C children overlaid according to the key in Table 2.
DISCUSSION
Our data are the first published measurements on DPF in the pediatric age group. The results of this study have demonstrated a wide variation in DPF between subjects of the same age, but the data have provided a means of estimating DPF from age for use in conventional NIRS. These values vary with wavelength and age, and have a 12-17% SD comparable to the variation of DPF measured by TOF techniques. The DPF depends upon the proportions of different scatterers and absorbers in tissue, and therefore the presence of bone, myelin, and muscle in the illuminated field. Even in a group of similarly aged subjects this may be a source of substantial variation. Other differences include geometrical and anatomical factors such as head shape and optode position. Ferrari et al.(31) have demonstrated an approximately 15% variation in DPF measured by TOF between the frontal and parietal areas of adult foreheads. We have shown a 0.8% variability between repeated measurements at one site on the same subject, which discounts the spread of results being due to machine error.
Although from this study we have recommended DPF for use with NIRS measurements in subjects of varying age, it is clear from the data that far better accuracy would be achieved by measuring individual path lengths at the time of each study. The machine described could be used to measure DPF in real time simultaneously with spectroscopy, which would be the most accurate method of performing quantitative studies, and would also reduce errors due to optode movement.
The change in DPF with wavelength is due to the relative contributions of scattering, absorption, and measurement geometry at different wavelengths. The variation with age is interesting. Modeling studies have shown that an increase in scattering or lowering in absorption can lead to an increase in DPF(32), as can an increase in head diameter at fixed optode spacing(33). Myelin is a major intracerebral scatterer, and it is possible to postulate that the increase in myelination with age is a major factor in the increase in DPF. The lower DPF in the 0-3-y-old group is consistent with postmortem findings in children that myelination is complete in about 50% of children by the end of the 2nd y of life(34). There are also observations that regional myelination changes continue to occur during adolescence(35, 36), suggesting a slow increase with age. The rapid increase in head diameter over the first 3 y of life will also contribute to the larger rate of increase of DPF in this age range. The significant increase of DPF with age in adulthood may be related to changes in cerebral blood volume. Results from PET studies have shown a drop in cerebral blood volume of 0.5% per year(37). As cerebral blood volume falls, the total amount of absorbing hemoglobin within the brain must fall, resulting in an increased number of photons being detected which have traveled greater distances through the brain. Thus the mean distance traveled and consequently the mean path length and DPF will be larger. The density and mineralization of cortical bone also increase gradually from birth to 18 y of age, but are then approximately constant throughout adult life(38), and this may also contribute to the more rapid rise of DPF in childhood.
Three children in group C are clear outliers. Two (Table 3, patients c and e) were neurologically abnormal after birth asphyxia and have a reduced DPF. It is feasible that these children may have delayed or reduced myelination or significantly increased cerebrospinal fluid space, both of which would alter the DPF. One (patient f) had Langerhans cell histiocytosis which can involve granulomatous lesions of the skull or brain, a possible reason for the higher DPF. These observations suggest that the measurement of DPF itself may provide information about pathology, and this could provide the basis for further studies.
Existing NIRS instrumentation (e.g. the NIRO500, Hamamatsu KK) uses a wavelength-dependent DPF which has been normalized to the value at 780 nm. As the wavelength dependence of DPF observed in this study is consistent with the values measured by the TOF technique for a group of six adults with a mean age of 28 y(21), the raw data have been interpolated to 780 nm, and a fifth curve was fitted to the resulting data to produce an equation for users of this machine:Equation

This study implies that DPF is an interesting variable in its own right, and we have shown that it can be quickly and noninvasively measured in a large group of people. These are the first measurements of DPF on children and adolescents. We have also provided equations for calculating DPF for use with conventional NIRS according to the age of the subject, although it is clear that ideally DPF should be measured for each subject at the time of each study.
Abbreviations
- DPF:
-
differential path length factor
- NIRS:
-
near infrared spectroscopy
- PRS:
-
phase resolved spectroscopy
- TOF:
-
time of flight
References
Jobsis FF 1977 Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198: 1264–1267.
Brazy JE, Darrell V, Lewis MD, Mitnick MH, Jobsis vander Vliet FF 1985 Noninvasive monitoring of cerebral oxygenation in preterm infants: preliminary observations. Pediatrics 75: 217–225.
Wyatt JS, Cope M, Delpy DT, Wray S, Reynolds EOR 1986 Quantification of cerebral oxygenation and hemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet 2: 1063–1066.
Cope M, Delpy DT 1988 System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infrared transillumination. Med Biol Eng Comput 26: 289–294.
Ferrari M, De Marchis C, Giannini I, Sideri G, Fieschi C, Carpi A 1986 Effects of carotid compression test on regional cerebral blood volume, haemoglobin oxygen saturation and cytochrome-c-oxidase redox level in cerebrovascular patients. Adv Exp Med Biol 200: 213–222.
Hampson NB, Camporesi EM, Stolp BW, Moon RE, Shook JE, Griebel JA, Piantodosi CA 1990 Cerebral oxygen availability by NIR spectroscopy during transient hypoxia in humans. J App Physiol 69: 907–913.
Villringer A, Planck J, Hock C, Scleinkofer L, Diragl U 1993 Near infrared spectroscopy (NIRS)-a new tool to study haemodynamic changes during activation of brain function in human adults. Neurosci Lett 154: 101–104.
Elwell CE, Cope M, Edwards AD, Wyatt JS, Delpy DT, Reynolds EOR 1994 Quantification of adult cerebral haemodynamics by near infrared spectroscopy. J Appl Physiol 77: 2753–2760.
Peebles DM, Edwards AD, Wyatt JS, Bishop AP, Cope M, Delpy DT, Reynolds EOR 1992 Changes in fetal cerebral hemoglobin concentration and oxygenation during labor measured by near-infrared spectroscopy. Am J Obstet Gynecol 166: 1369–1373.
Peebles DM, Edwards AD, Wyatt JS, Cope M, Delpy DT, Spenser JAD, Reynolds EOR 1994 Relation between frequency of uterine contractions and human fetal cerebral oxygen saturation studied during labour by near infrared spectroscopy. Br J Obstet Gynaecol 101: 44–48.
Tamura M 1991 Non-invasive monitoring of brain oxygen metabolism during cardiopulmonary bypass by near-infrared spectrophotometry. Jpn Circ J 55: 330–335.
Liem KD, Hopman JC, Kollee LA, Oeseburg B 1992 Assessment of cerebral oxygenation and hemodynamics by near infrared spectrophotometry during induction of ECMO: preliminary results. Adv Exp Med Biol 317: 841–846.
Fallon P, Roberts I, Kirkham FJ, Elliott MJ, Lloyd-Thomas A, Maynard R 1993 Cerebral hemodynamics during cardiopulmonary bypass in children using nearinfrared spectroscopy. Ann Thorac Surg 56: 1473–1477.
Edwards AD, Wyatt JS, Richardson CE, Delpy DT, Cope M, Reynolds EOR 1988 Cotside measurement of cerebral blood flow in ill newborn infants by near infrared spectroscopy. Lancet 2: 770:77771
Wyatt JS, Cope M, Delpy DT, Richardson CE, Edwards AD, Wray SC, Reynolds EOR 1990 Quantitation of cerebral blood volume in newborn infants by near infrared spectroscopy. J Appl Physiol 68: 1086–1091.
Cope M 1991 The development of a near infrared spectroscopy system and its application for non invasive monitoring of cerebral blood and tissue oxygenation in the newborn infant. PhD thesis, University of London
Delpy DT, Cope M, van der Zee P, Arridge SR, Wray S, Wyatt JS 1988 Estimation of optical pathlength through tissue from direct time of flight measurements. Phys Med Biol 33: 1433–1442.
Delpy DT, Arridge SR, Cope M, Edwards AD, Reynolds EOR, Richardson CE, Wray S, Wyatt JS, van der Zee P 1989 Quantitation of pathlength in optical spectroscopy. Adv Exp Med Biol 247: 41–46.
van der Zee P, Arridge SR, Cope M, Delpy DT 1990 The effect of optode positioning on optical pathlength in near infrared spectroscopy of brain. Adv Exp Med Biol 227: 79–84.
van der Zee P, Cope M, Arridge SR, Essenpreis M, Potter LA, Edwards AD, Wyatt JS, McCormick DC, Roth SC, Reynolds EOR, Delpy DT 1992 Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv Exp Med Biol 316: 143–153.
Essenpreis M, Elwell CE, Cope M, van der Zee P, Arridge SR, Delpy DT 1993 Spectral dependence of temporal point spread functions in human tissues. Appl Optics 32: 418–425.
Benaron DA, Lenox MA, Stevenson DK 1992 Two-D and three-D images of thick tissue using time constrained time of flight and absorbance (tc-TOFA) spectrophotometry. Proc SPIE 1641: 35–45.
Wyatt JS, Cope M, Delpy DT, van der Zee P, Arridge SR, Edwards AD, Reynolds EOR 1990 Measurement of optical pathlength for cerebral near infrared spectroscopy in newborn infants. Dev Neurosci 12: 140–144.
Cooper CE, Elwell CE, Meek JH, Matcher SJ, Wyatt SJ, Cope M, Delpy DT 1996 The noninvasive measurement of absolute cerebral deoxyhemoglobin concentration and mean optical path length in the neonatal brain by second derivative near infrared spectroscopy. Pediatr Res 39: 32–38.
Chance B, Maris M, Sorge J, Zhang MZ 1990 A phase modulation system for dual wavelength difference spectroscopy of hemoglobin deoxygenation in tissues. Proc SPIE 1204: 481–491.
Duncan A, Whitlock TL, Cope M, Delpy DT 1993 A multiwavelength wideband intensity modulated optical spectrometer for near infrared spectroscopy and imaging. Proc SPIE 1888: 248–257.
Benaron DA, Gwiazdowski S, Kurth CD, Steven J 1990 Delivoria-Papadopoulos, Chance B 1990 Optical path length of 754 nm and 816 nm light emitted into the head of infants. Proc IEEE BME 12: 1117–9.
Bolin F, Preuss LE, Taylor RC, Ference RJ 1989 Refractive index of some mammalian tissues using a fiber optic cladding method. Appl Optics 28: 2297–2302.
Arridge SR, Cope M, Delpy DT 1992 Theoretical basis for the determination of optical pathlengths in tissue: temporal and frequency analysis. Phys Med Biol 37: 1531–1560.
Duncan A, Meek JH, Tyszczuk L, Clemence M, Elwell CE, Cope M, Delpy DT 1995 Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol 40: 295–304.
Ferrari M, Wei Q, De Blasi R, Quaresima V, Zaccanti G 1993 Variability of human brain and muscle optical pathlength in different experimental conditions. Proc SPIE 1888: 466–72.
Pogue BW, Patterson MS 1994 Frequency-domain optical absorption spectroscopy of finite tissue volumes using diffusion theory. Phys Med Biol 39: 1157–1180.
Hiraoka M, Firbank M, Essenpreis M, Cope M, Arridge SR, van der Zee P, Delpy DT 1993 Simulation of light distribution in inhomogeneous tissue by a Monte Carlo method. Phys Med Biol 38: 1859–1877.
Kinney HC, Brody BA, Kloman AS, Gilles FH 1988 Sequence of central nervous system myelination in human infancy II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol 47: 217–234.
Benes FM, Turtle M, Khan Y, Farol P 1994 Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry 51: 477–484.
Holland BA, Haas DK, Norman D, Brant-Zawadzki M, Newton TH 1986 MRI of normal brain maturation. Am J Neuroradiol 7: 201–208.
Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S 1990 Cerebral blood flow, blood volume and oxygen utilization. normal values and effects of age. Brain 113: 27–47.
White DR, Widdowson EM, Woodard Q, Dickerson WT 1991 The composition of body tissues. Br J Radiol 64: 149–159.
Acknowledgements
The authors thank the staff at University College Hospital and the Great Ormond Street Hospital for Children, NHS Trust for their cooperation.
Author information
Authors and Affiliations
Additional information
Funded by a grant from the Wellcome Trust (Grant M/90/2790/DG/AE).
Rights and permissions
About this article
Cite this article
Duncan, A., Meek, J., Clemence, M. et al. Measurement of Cranial Optical Path Length as a Function of Age Using Phase Resolved Near Infrared Spectroscopy. Pediatr Res 39, 889–894 (1996). https://doi.org/10.1203/00006450-199605000-00025
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199605000-00025
This article is cited by
-
Open access dataset integrating EEG and fNIRS during Stroop tasks
Scientific Data (2023)
-
Chronotype predicts working memory-dependent regional cerebral oxygenation under conditions of normal sleep and following a single night of sleep extension
Scientific Reports (2023)
-
The Development of Cortical Responses to the Integration of Audiovisual Speech in Infancy
Brain Topography (2023)
-
Assessment of the mental workload of trainee pilots of remotely operated aircraft using functional near-infrared spectroscopy
BMC Neurology (2022)
-
Hypohydration alters pre-frontal cortex haemodynamics, but does not impair motor learning
Experimental Brain Research (2022)