Abstract
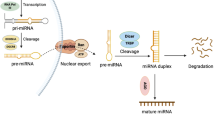
MicroRNAs (miRNAs) act as important epigenetic posttranscriptional regulators of gene expression. We aimed to gain more understanding of the complex gene expression regulation of endometrial receptivity by analyzing miRNA signatures of fertile human endometria. We set up to analyze miRNA signatures of receptive (LH + 7, n = 4) versus prereceptive (LH + 2, n = 5) endometrium from healthy fertile women. We found hsa-miR-30b and hsa-miR-30d to be significantly upregulated, and hsa-miR-494 and hsa-miR-923 to be downregulated in receptive endometrium. Three algorithms (miRanda, PicTar, and TargetScan) were used for target gene prediction. Functional analyses of the targets using Ingenuity Pathways Analysis and The Database for Annotation, Visualization and Integrated Discovery indicated roles in transcription, cell proliferation and apoptosis, and significant involvement in several relevant pathways, such as axon guidance, Wnt/β-catenin, ERK/MAPK, transforming growth factor β (TGF-β), p53 and leukocyte extravasation. Comparison of predicted miRNA target genes and our previous messenger RNA microarray data resulted in a list of 12 genes, including CAST, CFTR, FGFR2, and LIF that could serve as a panel of genes important for endometrial receptivity. In conclusion, we suggest that a subset of miRNAs and their target genes may play important roles in endometrial receptivity.
Similar content being viewed by others
References
Harper MJ. The implantation window. Baillieres Clin Obstet Gynaecol. 1992;6(2):351–371.
Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–1512.
Edwards RG. Clinical approaches to increasing uterine receptivity during human implantation. Hum Reprod. 1995;10(suppl 2):60–66.
Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27(2):170–207.
Sha AG, Liu JL, Jiang XM, et al. Genome-wide identification of micro-ribonucleic acids associated with human endometrial receptivity in natural and stimulated cycles by deep sequencing. Fertil Steril. 2011;96(1):150–155 e155.
Munro SK, Farquhar CM, Mitchell MD, Ponnampalam AP. Epigenetic regulation of endometrium during the menstrual cycle. Mol Hum Reprod. 2010;16(5):297–310.
Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004; 429(6990):457–463.
He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531.
Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10(2):94–108.
Creighton CJ, Benham AL, Zhu H, et al. Discovery of novel microRNAs in female reproductive tract using next generation sequencing. PLoS One. 2010;5(3):e9637.
Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125(6):1111–1124.
Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655.
Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–773.
Griffiths-Jones S. miRBase: microRNA sequences and annotation. Curr Protoc Bioinformatics. John Wiley & Sons, Inc., Hoboken, NJ, USA 2010;Chapter 12:Unit 12.9.1–10.
Miranda KC, Huynh T, Tay Y, et al. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217.
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009; 19(1):92–105.
Lam EW, Shah K, Brosens JJ. The role of microRNAs and FOXO transcription factors in cycling endometrium and cancer. J Endocrinol. 2012;212(1):13–25.
Hu SJ, Ren G, Liu JL, et al. MicroRNA expression and regulation in mouse uterus during embryo implantation. J Biol Chem. 2008; 283(34):23473–23484.
Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A. 2007;104(38):15144–15149.
Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod. 2010;82(4):791–801.
Revel A, Achache H, Stevens J, Smith Y, Reich R. MicroRNAs are associated with human embryo implantation defects. Hum Reprod. 2011;26(10):2830–2840.
Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262–263.
Altmäe S, Martinez-Conejero JA, Salumets A, Simon C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod. 2010;16(3):178–187.
Fernando RL, Nettleton D, Southey BR, Dekkers JC, Rothschild MF, Soller M. Controlling the proportion of false positives in multiple dependent tests. Genetics. 2004;166(1):611–619.
Krek A, Grun D, Poy MN, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37(5):495–500.
Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20.
Dennis G Jr, Sherman BT, Hosack DA, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):P3.
Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–284.
Riesewijk A, Martin J, van Os R, et al. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003; 9(5):253–264.
Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11(8):R90.
Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934.
Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A. 2008; 105(5):1608–1613.
Haouzi D, Mahmoud K, Fourar M, et al. Identification of new biomarkers of human endometrial receptivity in the natural cycle. Hum Reprod. 2009;24(1):198–205.
Mirkin S, Arslan M, Churikov D, et al. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod. 2005;20(8):2104–2117.
Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13(11):797–806.
Li R, Qiao J, Wang L, et al. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod Biol Endocrinol. 2011;9:29.
von Rango U, Classen-Linke I, Krusche CA, Beier HM. The receptive endometrium is characterized by apoptosis in the glands. Hum Reprod. 1998;13(11):3177–3189.
Zhang D, Lei C, Zhang W. Up-regulated monoamine oxidase in the mouse uterus during the peri-implantation period. Arch Gynecol Obstet. 2011;284(4):861–866.
Igci YZ, Arslan A, Akarsu E, et al. Differential expression of a set of genes in follicular and classic variants of papillary thyroid carcinoma. Endocr Pathol. 2011;22(2):86–96.
Pan SH, Chao YC, Hung PF, et al. The ability of LCRMP-1 to promote cancer invasion by enhancing filopodia formation is antagonized by CRMP-1. J Clin Invest. 2011;121(8):3189–3205.
Michel V, Bakovic M. The solute carrier 44A1 is a mitochondrial protein and mediates choline transport. FASEB J. 2009;23(8): 2749–2758.
Liu J, Lu WG, Ye F, et al. Hes1/Hes5 gene inhibits differentiation via down-regulating Hash1 and promotes proliferation in cervical carcinoma cells. Int J Gynecol Cancer. 2010;20(7):1109–1116.
Coffee RL Jr, Tessier CR, Woodruff EA 3rd, Broadie K. Fragile X mental retardation protein has a unique, evolutionarily conserved neuronal function not shared with FXR1P or FXR2P. Dis Model Mech. 2010;3(7–8):471–485.
Gaziel-Sovran A, Segura MF, Di Micco R, et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 2011;20(1):104–118.
Wang X, Zhang X, Ren XP, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/ reperfusion-induced cardiac injury. Circulation. 2010;122(13): 1308–1318.
Qian K, Hu L, Chen H, et al. Hsa-miR-222 is involved in differentiation of endometrial stromal cells in vitro. Endocrinology. 2009;150(10):4734–4743.
Aghajanova L, Hamilton AE, Giudice LC. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev Biol. 2008;19(2):204–211.
Driak D, Dvorska M, Svandova I, et al. Changes in expression of some apoptotic markers in different types of human endometrium. Folia Biol (Praha). 2011;57(3):104–111.
Altmäe S, Reimand J, Hovatta O, et al. Research resource: interactome of human embryo implantation: identification of gene expression pathways, regulation, and integrated regulatory networks. Mol Endocrinol. 2012;26(1):203–217.
Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction. 2010;139(4):697–704.
Chen Q, Zhang Y, Lu J, et al. Embryo-uterine cross-talk during implantation: the role of Wnt signaling. Mol Hum Reprod. 2009;15(4):215–221.
Koler M, Achache H, Tsafrir A, Smith Y, Revel A, Reich R. Disrupted gene pattern in patients with repeated in vitro fertilization (IVF) failure. Hum Reprod. 2009;24(10):2541–2548.
Liu Y, Kodithuwakku SP, Ng PY, et al. Excessive ovarian stimulation up-regulates the Wnt-signaling molecule DKK1 in human endometrium and may affect implantation: an in vitro coculture study. Hum Reprod. 2010;25(2):479–490.
Kao LC, Tulac S, Lobo S, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143(6):2119–2138.
Horcajadas JA, Pellicer A, Simon C. Wide genomic analysis of human endometrial receptivity: new times, new opportunities. Hum Reprod Update. 2007;13(1):77–86.
Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450(7170):721–724.
Feng Z, Zhang C, Kang HJ, et al. Regulation of female reproduction by p53 and its family members. FASEB J. 2011;25(7): 2245–2255.
Massuto DA, Kneese EC, Johnson GA, et al. Transforming growth factor beta (TGFB) signaling is activated during porcine implantation: proposed role for latency-associated peptide interactions with integrins at the conceptus-maternal interface. Reproduction. 2010;139(2):465–478.
Genbacev OD, Prakobphol A, Foulk RA, et al. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299(5605):405–408.
Dominguez F, Yanez-Mo M, Sanchez-Madrid F, Simon C. Embryonic implantation and leukocyte transendothelial migration: different processes with similar players? FASEB J. 2005; 19(9):1056–1060.
Dahiya N, Sherman-Baust CA, Wang TL, et al. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS One. 2008;3(6):e2436.
Boren T, Xiong Y, Hakam A, et al. MicroRNAs and their target messenger RNAs associated with endometrial carcinogenesis. Gynecol Oncol. 2008;110(2):206–215.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altmäe, S., Martinez-Conejero, J.A., Esteban, F.J. et al. MicroRNAs miR-30b, miR-30d, and miR-494 Regulate Human Endometrial Receptivity. Reprod. Sci. 20, 308–317 (2013). https://doi.org/10.1177/1933719112453507
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719112453507