Abstract
Non-invasive brain stimulation (NIBS) is emerging as a promising rehabilitation tool for a number of neurodegenerative diseases. However, the therapeutic mechanisms of NIBS are not completely understood. In this review, we will summarize NIBS results in the context of brain imaging studies of functional connectivity and metabolites to gain insight into the possible mechanisms underlying recovery. We will briefly discuss how the clinical manifestations of common neurodegenerative disorders may be related with aberrant connectivity within large-scale neural networks. We will then focus on recent studies combining resting-state functional magnetic resonance imaging with NIBS to delineate how stimulation of different brain regions induce complex network modifications, both at the local and distal level. Moreover, we will review studies combining magnetic resonance spectroscopy and NIBS to investigate how microscale changes are related to modifications of large-scale networks. Finally, we will re-examine previous NIBS studies in dementia in light of this network perspective. A better understanding of NIBS impact on the functionality of large-scale brain networks may be useful to design beneficial treatments for neurodegenerative disorders.
Introduction
Dementia is a chronic syndrome characterized by a progressive decline in cognition, behavior, and everyday activities that affects mainly older people and, with the population aging at a fast rate worldwide, its burden is destined to increase dramatically [1]. To date, no pharmacological treatment is available to prevent or cure dementia, thus highlighting the urgent need for new, effective, therapeutic strategies.
In recent years, non-invasive brain stimulation (NIBS) techniques, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) have been developed and are currently under investigation in patients with dementia [2]. During TMS, transient rapid-changing magnetic fields are used to induce secondary electric currents in the underlying cortical surface, which, in turn, trigger neuronal action potentials [3]. Single-pulse TMS is commonly employed to study brain functioning, while repetitive TMS (rTMS) is the preferred approach to induce long-lasting changes in brain activity. By contrast, during tDCS, a weak electrical current is directly applied to the scalp to modulate neuronal membrane potentials without directly inducing synchronized neuronal discharge [4]. Both rTMS and tDCS can have excitatory or inhibitory effects, depending on the frequency (rTMS) and the polarity (tDCS), respectively [5]. Low-frequency (< 1 Hz) rTMS is thought to have inhibitory effects, high-frequency stimulation (> 5 Hz) excitatory effects; tDCS induces neural hyperpolarization under the cathode and depolarization under the anode, respectively, reducing/increasing the responsiveness of the target neurons to the ongoing afferent brain activity [6, 7]. Recently, theta-burst stimulation (TBS), a variant of TMS, has received increasing interest thanks to its ability to induce long-lasting changes just after a few minutes of application of burst of high frequency stimulation, delivered either as a continuous (cTBS) or intermittent (iTBS) train [8]. The former protocol is characterized as being “inhibitory” and the latter “excitatory,” according to the changes produced in motor evoked potentials size [9].
Although preliminary evidence indicates promising effects of NIBS in neurodegenerative diseases [10, 11], there are still several barriers to the application of these techniques in the clinical practice. First, the mechanisms by which NIBS exerts its clinical effects are yet to be determined. Specifically, it is unclear how the transition of NIBS effects from the cellular to the mesoscopic, macroscopic, and behavioral levels occurs [12]. In the case of dementia, this issue is complicated by the presence of aging- and disease-related phenomena (e.g., atrophy, reduced intrinsic plasticity) that may importantly mitigate the impact of NIBS. Furthermore, NIBS after-effects are highly dependent on a variety of parameters (e.g., the localization of TMS coil or tDCS electrodes, intensity, and duration) [13], all aspects for which there is no consensus yet. Finally, NIBS is usually applied focusing on the behavioral effects but overlooking the possible underlying biological processes. However, it is now well established that the major dementias are linked to specific molecular pathologies and that these pathologies affect specific large-scale networks [14].
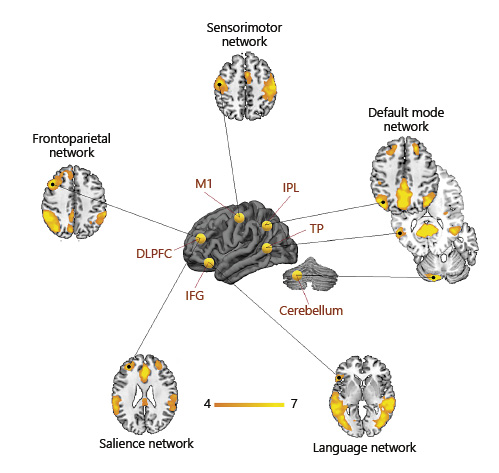
In the past 10 years, resting-state functional MRI (rs-fMRI) identified neural networks associated with specific cognitive and motor-sensory functions, and the neurodegenerative disorders associated with a dysfunction of these networks (Fig. 1). Studies suggest that memory is sustained by two networks, the default mode network (DMN) and the limbic network [15]. Neocortical networks such as the frontoparietal (FPN), visual, and language networks underlie executive, visuospatial, and language abilities, respectively [16]. Moreover, the so-called salience network (SN) is involved in social behavior, emotion regulation, and self-awareness [17].
Neural networks associated with specific cognitive and sensorimotor functions and the associated neurodegenerative disorders. Maps were extracted through independent component analysis from a sample of 20 healthy subjects.
Neural networks associated with specific cognitive and sensorimotor functions and the associated neurodegenerative disorders. Maps were extracted through independent component analysis from a sample of 20 healthy subjects.
There is increasing evidence that neurodegenerative diseases affect specific networks, leading to the hypothesis of “molecular nexopathy” models of disease progression (i.e., the conjunction mechanism between molecular pathology and neural network disruption) [18]. This assumption is supported by several studies reporting a close correspondence between network disruption, molecular disease, and clinical symptoms [14]. In typical late-onset Alzheimer’s disease (AD), consistently with the core symptom (i.e., memory impairment), the DMN and the limbic network show selective vulnerability [19]. Conversely, early-onset AD variant preferentially affects neocortical functions such as language, executive and visuospatial abilities, showing aberrant connectivity in neocortical networks sustaining these functions [20]. Within the frontotemporal dementia spectrum, primary progressive aphasia (PPA), a disorder characterized by language impairment [21], shows disruption of the language network [22], while the behavioral variant of frontotemporal dementia (bvFTD), which is characterized by a range of behavioral disturbances [23], is associated with the disruption of the SN [24]. Similarly, posterior cortical atrophy, a condition characterized by predominant visuospatial dysfunctions [25] is associated with lower connectivity in the higher visual network [20]. Network abnormalities in Parkinson’s disease (PD) and corticobasal syndrome (CBS), two disorders of the motor spectrum, seem to involve the cerebello-thalamo-cortical and sensorimotor networks, respectively [14, 26]. While this evidence points to a clear correspondence between functional networks and syndrome-specific core symptoms, network dysfunction is not limited to a single circuit but generally involves several networks [17].
The disruption of large-scale neural networks might provide an intermediate link between pathology and clinical symptoms for neurodegenerative dementias. Therefore, brain stimulation of affected neural networks may offer a novel therapeutic strategy. To date, most of NIBS clinical interventions have focused on the effect of modulation on single brain areas, possibly overlooking more widespread effects of stimulation over distal areas or large-scale networks. This approach provides a fragmented view of NIBS effects and prevents a clear understanding of the NIBS mechanisms underlying cognitive improvement in dementia. Conversely, a “network perspective” would consider not only the effects in targeted brain regions but within areas integrated in complex, distributed, large-scale neural networks [27], and this approach may better capture the neural correlates of behavioral changes after stimulation.
This review aims to provide a network perspective on NIBS interventions for healthy individuals and patients with dementia. In the next paragraph, we will review studies combining NIBS with functional imaging in cognitively healthy adults, showing how NIBS induces both local and distal connectivity changes. Subsequently, we will investigate the possible mechanisms underlying NIBS effects by reviewing studies in healthy combining stimulation with proton magnetic resonance spectroscopy (1H MRS), a MRI modality that enables to measure the level of specific neurotransmitters and neurometabolites underlying neuronal communication. Finally, we will retrospectively evaluate previous NIBS clinical applications in the most common neurodegenerative disorders in the light of the above considerations.
Local and Distal Connectivity Effects of NIBS
To date, several studies have combined NIBS with rs-fMRI in young healthy subjects to investigate the modulatory effects of NIBS on brain networks (Table 1). Overall, these studies consistently reported that NIBS alters connectivity in the target brain region (local effect) but also in remote regions interconnected within the stimulated area (distal effect). These studies, reviewed below, are classified according to the target of stimulation (Fig. 2).
Common targets of NIBS reported in the literature (yellow spheres on 3D brain) are major nodes of large-scale functional networks. Each target can be used as a seed to derive functionally related (i.e., synchronous) regions of the brain. Stimulation of specific brain regions results in both local and distal functional connectivity modulation. Orange-yellow colors on axial slices represent the intrinsic functional connectivity degree (z score) of the major large-scale networks, extracted through independent component analysis from a sample of 20 healthy subjects.
Common targets of NIBS reported in the literature (yellow spheres on 3D brain) are major nodes of large-scale functional networks. Each target can be used as a seed to derive functionally related (i.e., synchronous) regions of the brain. Stimulation of specific brain regions results in both local and distal functional connectivity modulation. Orange-yellow colors on axial slices represent the intrinsic functional connectivity degree (z score) of the major large-scale networks, extracted through independent component analysis from a sample of 20 healthy subjects.
Sensorimotor and Auditory Cortex
Studies investigating NIBS effects over the sensorimotor cortex generally used the primary motor region (M1) as the target of stimulation, consistently showing increased connectivity after atDCS. Increased functional connectivity (FC) was found both in regions proximal to the electrode [30, 34, 38] and in distal regions belonging to the sensorimotor network [31, 36, 39]. Similarly, studies using graph theory, an approach taking into account network topology and synchronization between brain hubs [64], suggested a network-specific enhancement of connectivity following atDCS [32, 33, 35, 37]. Taken together, these results suggest that atDCS targeting the motor cortex induce increased connectivity, exerting both local and distal effects within the sensorimotor network. However, in elderly atDCS seems to decrease FC of motor network suggesting a differential effect of atDCS due to age [29, 30], although the behavioral significance of these changes is not yet clear. The relationship between ctDCS and functional changes is also unclear, since variables effects have been reported to date (i.e., no change, increased, decreased FC) [28-30, 34].
Only one study has used TMS to investigate the relationship between polarity (high/low frequency) and FC changes in the motor network [40]. The study targeted the left M1 and reported reduced FC following excitatory TMS, while inhibitory TMS increased FC. This pattern differs from that reported for tDCS and suggests that the mechanism of action of the two methodologies might not be directly comparable.
Two studies have used TBS to test the effect of motor cortex inhibition. Inhibitory cTBS over the somatosensory cortex (S1) reduced FC between the stimulated region and functionally connected motor network regions [42]. Similarly, cTBS over the bilateral auditory cortex reduced FC within the auditory network [41]. These studies suggest that cTBS may be more effective than ctDCS in inhibiting FC.
Frontal and Parietal Cortex
Studies targeting the dorsolateral prefrontal cortex (DLPFC), a key hub for executive functions, working memory and reasoning, consistently reported increased FC within the FPN after atDCS [43, 47, 48]. Studies targeting the temporo-parietal cortex, a key region of the DMN, reported relatively consistent changes within this network. Increased FC within the DMN was observed after 3 atDCS sessions targeting the right temporo-parietal cortex, and these changes were associated with better memory recall performance [55]. Similarly, Clemens et al. [57] reported increased FC within the anterior DMN after a single atDCS targeting the right angular gyrus, although divergent effects were reported for DMN posterior hubs [56, 57]. These preliminary results suggest that atDCS over DLPFC and parietal cortex may be effective in modulating specific neural networks relying over these cortical hubs, the FPN and the DMN, respectively. Nevertheless, connectivity changes were also observed in other neural networks: frontal stimulation affected FC within the DMN [43, 47], the language network [44], and subcortical nuclei [36, 46]. Moreover, frontal stimulation resulted in interactions between FPN and DMN FC [47]. Similarly, parietal stimulation effects were not limited to the DMN but also involved the FPN and SN [57, 58].
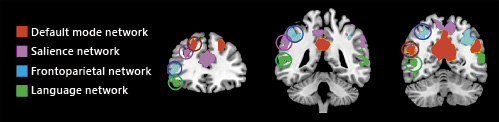
The reason for these internetwork changes following tDCS remains to be elucidated. Between-networks effects may be related to accidental (co)stimulation of directly adjacent brain regions, which in turn may cause multiple network effects and even unexpected negative effects on cognitive outcomes [59]. To resolve this issue, rs-fMRI could be used to identify non-overlapping hubs from specific networks. Indeed, procedures of signal decomposition such as independent component analysis (ICA) enable to extract distinct (spatially non-overlapping) nodes for each network (Fig. 3). However, some degree of overlap between networks might be unavoidable. Macroscopic anatomical brain connectivity studies suggest a so-called “rich club” organization of the brain, whereby highly interconnected nodes show a strong tendency to connect with other highly connected nodes, thus resulting in numerous between-network connections [65]. While the sensorimotor network shows a relatively small proportion of rich club nodes (6–8%), the DMN, SN, and FPN show considerably more rich club nodes (up to 23%) [66]. Thus, although different DLPFC and posterior parietal subregions are exclusively part of a network, they may be highly interconnected, and this observation may, in part, explain the different neuromodulatory effects reported in the studies above. Conversely, the lower proportion of rich club within the sensorimotor network may explain the higher consistency between studies targeting motor cortex with atDCS electrodes and the relatively selective effect on this network.
Non-overlapping hubs for the major cognitive networks (language, frontoparietal, salience, and default mode networks). Maps were extracted through independent component analysis from a sample of 20 healthy subjects and overlaid into the MNI template. Circles highlight the spatial localization of non-convergent dorsal frontal and parietal hubs of each functional network.
Non-overlapping hubs for the major cognitive networks (language, frontoparietal, salience, and default mode networks). Maps were extracted through independent component analysis from a sample of 20 healthy subjects and overlaid into the MNI template. Circles highlight the spatial localization of non-convergent dorsal frontal and parietal hubs of each functional network.
To date, only one study assessed local and distal connectivity effects following frontal stimulation in elderly [45]. After atDCS over the left IFG, participants significantly improved performance up to the level of younger controls, along with a “normalization” of network connectivity [45]. This finding provides the first evidence that NIBS may be suited to modulate brain networks connectivity in elderly populations.
Studies using TMS reported more variable findings. Three studies used TMS [49-51] and 3 TBS [52-54] to stimulate the DLPFC. Two studies showed coherent findings, i.e. increased FC within the frontal hub of the FPN after excitatory TMS [50], and reduced FPN after inhibitory TBS [54], while the others did not report differences in FPN connectivity after TMS/TBS [49, 51], or reported an inverted direction of effects (i.e., increased FC after inhibitory or decreased FC after excitatory TBS) [52, 53]. Similarly, Eldaief et al. [60] using rs-fMRI at baseline to identify individual DMN parietal targets, reported opposite effects according to the frequency of TMS: low-frequency stimulation increased DMN FC, while high-frequency stimulation decreased it [60]. However, a subsequent study reported the opposite effect, i.e. inhibitory cTBS of the precuneus reduced DMN FC [61]. While the reason for the differences between TMS and TBS effects is unclear, it seems that the mechanism of action of the two techniques is different. Moreover, differences in methodology (i.e., stimulation parameters) may explain, at least in part, this discrepancy, as well as in the target (IPL vs. precuneus) and coil positioning and orientation, which are known to affect the neurophysiological response to TMS [67].
Cerebellum
Modulation of cognitive networks was also observed after the stimulation of cerebellar hubs, which are traditionally associated with motor functions. As in the study by Eldaief et al. [60], Halko et al. [62] used the baseline rs-fMRI of participants to localize the cerebellar node of the DMN. Stimulation through iTBS of the DMN-cerebellum node resulted in increased FC of cortical nodes of the DMN. Targeting the midline cerebellum, which is not part of the DMN, increased FC of the dorsal attention network [62]. Rastogi et al. [63] found reduced FC in frontal and parietal cognitive regions after cTBS of the crus I of the lateral cerebellum. These results confirm the modulatory effect of cerebellar stimulation on large-scale cognitive networks and the opposite effect of excitatory and inhibitory TBS on connectivity.
Mechanisms of Network Modulation: Brain Neurometabolites
The investigation of brain neurotransmitters in relation to NIBS effects can provide insight into the mechanisms of action of NIBS. Indeed, neuronal plasticity depends upon a complex balance between excitatory and inhibitory neurotransmitters: glutamate (Glu) is the main brain excitatory neurotransmitter, while the γ-amino butyric acid (GABA) is the main inhibitory neurotransmitter [68]. Pharmacological studies indicate that excitatory changes induced by atDCS are Glu dependent but are also involving a reduction in GABA transmission [69], whereas the inhibitory effects induced by ctDCS seem to involve a reduction in Glu transmission [70]. Glutamatergic/GABAergic involvement is also observed in TMS-induced excitatory and inhibitory effects on the brain, which have been linked to long-term potentiation and long-term depression, respectively [71].
Neuroimaging can offer an alternative approach to pharmacological studies to study these mechanisms. The concentration of GABA and glutamatergic metabolites, Glu/glutamine (Glx), can be measured reliably in vivo with 1H MRS [72], using “Spectral editing” techniques [73]. In recent years, these 1H MRS techniques have been increasingly used in conjunction with NIBS and rs-fMRI in healthy adults (Table 2). The areas of interest (rs-fMRI) or targeting (NIBS) have been principally studied by using a single-voxel MRS technique and, to our best knowledge, the measurements of the GABA/Glx published are mainly relative (ratio), not absolute concentrations (Table 2).
Non-invasive brain stimulation studies in healthy controls using 1H MRS-derived metabolites as outcome measures

The majority of these studies investigated neurometabolite changes in the motor cortex in young adults, reporting a consistent reduction of GABA after atDCS [31, 70, 75, 76], except for Tremblay et al. [77]. The above finding was generally coupled with increased FC over the motor network [31, 39], indicating that these connectivity changes may be driven by a decrease in the inhibitory neurotransmitter. Indeed, Stagg et al. [76] estimated a decrease of around 12% of GABA levels in the motor cortex following motor atDCS and an inverse correlation with motor network FC [39]. Conversely, the effects of ctDCS on brain metabolites levels are less clear, one study reporting no effect [75], and one decreased GABA and Glu levels [70]. Age may modulate the effects of NIBS on brain neurometabolites. One study to date has assessed this topic. Antonenko et al. [29] reported a reduction of GABA concentrations following atDCS together with a decreased FC in the sensorimotor cortex in elderly. This pattern is different from that observed in young adults [30, 31, 39]. Stratification of the elderly sample into younger (< 63 age) and older (> 63 age) revealed a differential effect of atDCS-induced GABA modulation and network coupling: in younger, lower GABA correlated with higher sensorimotor strength (i.e., the same pattern observed in young adults), while in older the correlation was reversed [29]. Therefore, functional network reorganization may follow different trajectories in young and elderly, and the physiological significance of these changes (whether indicative of higher neuronal efficiency at different ages or impaired patterns in elderly) remains to be elucidated.
Two studies investigated the effect of atDCS over the parietal cortex, reporting increased Glx concentrations [58, 74], which suggests an increase in excitation. Notably, this is different from the effect reported in the motor cortex, which suggests a decrease in inhibition. However, these considerations are very preliminary, since the above studies did not measure GABA concentrations [58, 74]. The only study specifically investigating parietal after-effects of excitatory stimulation over both GABA and Glx used TBS and was aimed at DMN stimulation through the left IPL [78]. Inhibitory TBS induced distal GABA increases in the posteromedial DMN areas, with no local change in GABA or Glu [78]. Moreover, increments in distal GABA concentration were significantly related to baseline FC between the stimulated site and the posteromedial cortex, while nonsignificant Glx modulation after iTBS was inversely related to FC, suggesting that baseline connectivity patterns predict neurotransmitter modulation in distal areas.
Only one study to date has investigated the effect of frontal (DLPFC) cortex stimulation on brain metabolites. Iwabuchi et al. [53] reported a significant reduction in the GABA/Glx ratio following iTBS (suggesting reduced inhibition and/or increased excitation), although neither GABA nor Glx concentrations alone showed significant changes.
This preliminary evidence supports the idea that NIBS effects on functional networks may stem from modulation of GABAergic and glutamatergic pathways, in line with neuroimaging studies showing an association between metabolite levels and neural network connectivity [79, 80]. However, future studies in larger samples are needed to disentangle the variability of NIBS-induced effects and the impact on neural networks of neurometabolite modulation.
NIBS and Dementia
NIBS is increasingly used to improve cognitive/behavioral deficits in neurodegenerative dementias or as a possible cognitive enhancer in preclinical stages (Table 3). A better understanding of the clinical effects of NIBS may be gained focusing on the complex interactions between the stimulation target and the associated brain networks. In this section, we will review previous NIBS studies carried out in dementia focusing on this specific aspect.
Alzheimer’s Disease
For studies with AD patients, roughly half of studies employed tDCS as the stimulation tool [81-87], the other using TMS [88-94]. Moreover, the majority of the studies have focused on memory, the core clinical symptom. Ten studies were designed as multiple session paradigm [81, 83, 84, 86-88, 90, 92-94], and a cognitive training was combined with the stimulation protocol in 3 of the brain stimulation studies on AD [84, 93, 94].
Memory improvement was reported in 3 out of 5 studies targeting the temporal region [81-83, 85, 91]. According to the study by Antonenko et al. [55] in healthy older subjects, atDCS targeting the temporo-parietal cortex resulted in better memory, and this improvement was associated with increased DMN connectivity. Considering the role of DMN in supporting memory [15], it is conceivable that memory improvement following atDCS may be mediated by restoration of FC within the DMN. While the above studies did not investigate NIBS-related DMN changes, a recent investigation using TMS and EEG provide some preliminary support to this view. In a sample of 14 AD patients, Koch et al. [92] reported improved episodic memory and increased EEG connectivity within the DMN after excitatory TMS over the precuneus. This region, part of the medial parietal component of the DMN, displays aberrant connectivity in AD [19]. If confirmed with MRI approaches, this observation may indicate that memory improvement in AD following parietal excitatory stimulation may be mediated by DMN modulation.
Other studies in AD have targeted the frontal cortex, specifically the DLPFC, providing contrasting results in terms of memory benefits [82, 84, 87]. While one study reported a significant modulatory effect of atDCS on visual recognition memory [82], the other two did not report a stimulation benefit on memory tests [84, 87]. The type of treatment protocol used could be responsible of the different results reported, since one of those investigations tested whether combining NIBS with cognitive training leads to a pronounced enhancement of training effects [84]. On the other hand, DLPFC modulation seems effective in improving global cognition, language, and attention abilities in AD patients [86, 88, 89, 91]. Accordingly, the beneficial effects on non-memory abilities of NIBS over the frontal cortex may be related to modulation of other (non-DMN) networks.
Subjective Memory Complaints and Mild Cognitive Impairment
Manenti et al. [95] investigated the effect of atDCS on the DLPFC in individuals with subjective memory complaints, a condition at greater risk of AD [116]. Their findings showed that a single session over the DLPFC was sufficient to strengthen episodic memory up to 1 month, relative to sham stimulation [95]. Similarly, DLPFC modulation showed beneficial memory effects in mild cognitive impairment (MCI) patients [97, 98], although these positive effects were obtained using different stimulation protocols (i.e., 10 excitatory sessions vs. 1 inhibitory session). In a sham-controlled study, Meinzer et al. [96] demonstrated that atDCS improved language performance in MCI patients after IFG stimulation. Connectivity analysis revealed a restoration of abnormal network configuration in the active group compared to sham condition in areas functionally connected to the stimulated IFG [96] and involved in language abilities [117].
Primary Progressive Aphasia
NIBS interventions in PPA are aimed at restoring language deficits. To date, all NIBS studies were carried out using tDCS and generally reported language enhancement. While there is some variability among studies in the target region, the majority stimulated areas involved in language, such as the left perisylvian cortex [102, 104], left anterior temporal pole [105], left frontotemporal cortex [101], but also areas within the dorsal frontal cortex (left DLPFC and IFG) [99, 100, 106]. Interestingly, 7 of the 8 studies were designed as multiple-session paradigm [99-104, 106] and 4 of them combined a cognitive training with the stimulation protocol [100, 102, 104, 106].
The above studies did not collect surrogate measures of neuronal changes; thus, the mechanism underlying cognitive improvement cannot be elucidated; however, one recent study suggests that language improvement might be driven by modulation of the language network but also by between-network changes. In a relatively large cohort of PPA patients (n = 24) treated in a crossover design, Ficek et al. [100] found that atDCS over the left IFG combined with speech and language interventions was more beneficial than sham combined with speech and language interventions. Significant tDCS effects in FC were observed between the stimulated area and the language network hubs and between the language network and the DMN (i.e., reduced connectivity), and these changes correlated with improvement in language scores. These results are in line with similar decreases in connectivity observed after tDCS over the left IFG in aging and MCI [45, 96], suggesting that language improvement might be mediated by uncoupling between networks.
Behavioral Variant Frontotemporal Dementia
To our knowledge, only 3 studies have investigated NIBS effect in bvFTD patients. Only one study used multiple sessions [109] and none combined a cognitive training with the stimulation protocol. In all cases, the target region was the frontal cortex (dorsal or medial prefrontal cortex). Huey et al. [107] did not report any improvement in a sample of 10 FTD (9 bvFTD and 1 PPA) patients following a single atDCS stimulation targeting the prefrontal cortex. However, the choice of the outcome (language rather than behavioral domain) might have not been sensible to detect NIBS changes in this population. Recently, 2 studies performed NIBS targeting the frontal cortex with tDCS [108] or TMS [109], showing improvement in social cognitive ability [108], global cognition, and behavioral symptoms [109]. However, none of these studies collected surrogate imaging markers and one can only speculate on the possible networks modulating these effects. Based on previous studies, enhancement of behavioral symptoms might be effective through SN modulation, while modulation of social cognition activity might require targeting the SN, DMN, and attention network.
Overall, these results are promising for the treatment of bvFTD, for which therapeutic options are lacking. However, future studies should directly investigate NIBS-modulatory effects on neural networks involved in FTD, such as the SN, DMN, and attention network.
Other Neurological Conditions
In the last years, NIBS studies have been conducted in neurological disorders such as vascular dementia (VaD), Lewy body dementia (LBD), PD and CBS.
In VaD, extensive white matter lesions result in global cognitive slowing and frontal-executive dysfunctions [118]. André et al. [110] investigated the effect of four daily sessions of 2 mA anodal or sham at-home tDCS applied to the left DLPFC in 21 patients with mild VaD. The left DLPFC was chosen as the target given its relevance in the modulation of executive functions. As expected, atDCS was able to improve the performance on executive tests [110].
Executive dysfunction is also present in PD and in LBD from the early stages [119]. Two independent studies in PD with MCI modulating the DLPFC however showed contrasting results. Biundo et al. [112] did not report improvements in executive functions after atDCS combined with cognitive training. In contrast, Manenti et al. [114], testing 2 weeks of atDCS combined with physical training, observed significant improvement of the Parkinson’s disease cognitive rating test and verbal fluency, with a significant effect also at follow-up [114]. One study in DLB reported improvement in attention functions after 1 session of tDCS in a sample of LBD [113].
These studies generally used the DLPFC as a target, due to the key role of this region in executive functions. Studies in healthy subjects suggest that the executive function benefits may be mediated by FPN modulation [43, 47, 48]. Therefore, DLPFC modulation through NIBS may be an effective strategy to modulate executive functions in all those neurological disorders suffering from executive dysfunction. While the above studies are promising, they are still preliminary, and replication in larger, placebo-controlled trials is needed. Moreover, the effect of alternative approaches has yet to be investigated. For example, PD is primarily a disease of the cerebello-thalamo-cortical network, and assessing these networks might help to identify novel targets to inform on the mechanisms of cognitive/motor improvement.
CBS is a progressive neurodegenerative disease characterized by marked movement deficits and cognitive dysfunction and is associated with atrophy of the motor (parietal) cortex [120]. To date, 2 studies have investigated the effects of atDCS targeting the left parietal cortex and reporting improved naming abilities and apraxia after stimulation [111, 115]. These studies used the parietal cortex as a target, due to the key role of this region in frontoparietal network. While the above studies could pave the way to useful rehabilitation techniques in these patients, they are still preliminary, and replication in larger, placebo-controlled trials is needed. Moreover, the effect of alternative approaches such as the stimulation of sensorimotor network, which is generally impaired in CBS pathology, has yet to be investigated. Further investigations might help to identify novel targets to inform on the mechanisms of cognitive/motor improvement in CBS.
Conclusions
NIBS has the potential to modulate the functional organization of brain large-scale networks. The reviewed literature indicates that NIBS affects both local and distal regions. Studies focusing on motor cortex consistently showed modulation of the sensorimotor network, while the effect of parietal and frontal cortex stimulation was more heterogeneous, although preliminary evidence seems to point to an involvement of the DMN and FPN, respectively. Importantly, the stimulation of regions belonging to cognitive networks (e.g., frontal and parietal cortex) demonstrated off-target effects (e.g., between-network changes), which were not observed for the sensorimotor network. Overall, findings suggest that modulation of neural networks following NIBS does not rely on a simple 1: 1 relationship between brain target and a given network, but several factors may interact to influence the results. For instance, age is one of the most important variables that may influence functional modulation.
Microscale changes observed with the 1H MRS technique may help to better understand the mechanisms underlying functional modulation, such as the influence of GABA versus glutamate on the observed effects, although the limited number of studies available thus far does not allow to reach univocal conclusions. Again, studies investigating the effect on brain metabolites reported relatively consistent findings when modulating the motor cortex, while findings were more complex when studies modulated cognitive networks.
In conclusion, in neurodegenerative disorders, the association between cognitive/clinical deficits and network dysfunction supports a “network” approach targeting neural networks linked with the clinical phenotype. The combination of NIBS with neuroimaging is a promising approach to better understand the mechanisms of neuromodulation and to design targeted interventions for neurocognitive diseases.
Statement of Ethics
All data included were collected according to the Helsinki Declaration and approved by the local ethics committee of the IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy. All participants signed written informed participation consent.
Disclosure Statement
The authors declare no conflicts of interest.
Funding Sources
This work was supported by the Italian Ministry of Health (Giovani Ricercatori grant GR2011-02349787 and Ricerca Corrente).