Abstract
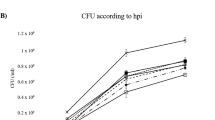
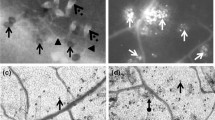
Ralstonia solanacearum, a soil-borne bacterium causes bacterial wilt, is a lethal disease of eggplant (Solanum melongena L.). However, the first line of defense mechanism of R. solanacearum infection remains unclear. The present study focused on the role of induced H2O2, defense-related enzymes of ascorbate-glutathione pathway variations in resistant and susceptible cultivars of eggplant under biotic stress. Fifteen cultivars of eggplant were screened for bacterial wilt resistance, and the concentration of antioxidant enzymes were estimated upon infection with R. solanacearum. A quantitative real-time PCR was also carried out to study the expression of defense genes. The concentration of H2O2 in the pathogen inoculated seedlings was two folds higher at 12 h after pathogen inoculation compared to control. Antioxidant enzymes of ascorbate-glutathione pathway were rapidly increased in resistant cultivars followed by susceptible and highly susceptible cultivars upon pathogen inoculation. The enzyme activity of ascorbate-glutathione pathway correlates by amplification of their defense genes along with pathogenesis-related protein-1a (PR-1a). The expressions of defense genes increased 2.5−3.5 folds in resistant eggplant cultivars after pathogen inoculation. The biochemical and molecular markers provided an insight to understand the first line of defense responses in eggplant cultivars upon inoculation with the pathogen.
Similar content being viewed by others
Abbreviations
- ANOVA:
-
analysis of variance
- APX:
-
ascorbate peroxidase
- BYMV:
-
Bean yellow mosaic virus
- DHAR:
-
dehydroascorbate reductase
- GR:
-
glutathione reductase
- H2O2 :
-
hydrogen peroxide
- HS:
-
highly susceptible
- MDHAR:
-
monodehydroascorbate reductase
- PR:
-
pathogen-related
- R:
-
resistance
- ROS:
-
reactive oxygen species
- S:
-
susceptible
References
FAO, Food and Agriculture Organization of United Nations, Agriculture Data, 2013. http://www.faostat.com.
Varma, R.K. and Bhale, M.S., Microorganisms for the management of Phomopsis blight and fruit rot of eggplant caused by Phomopsis vexans (Sacc. & Syd.) Harter. L., J. Mycopathol. Res., 2010, vol. 48, pp. 245–249.
Avinash, P. and Umesha, S., Identification and genetic diversity of bacterial wilt pathogen in brinjal, Arch. Phytopathol. Plant Prot., 2013, vol. 47, pp. 398–406.
Vanitha, S.C., Niranjana, S.R., and Umesha, S., Role of phenylalanine ammonia lyase and polyphenol oxidase in host resistance to bacterial wilt of tomato, J. Phytopathol., 2009, vol. 157, no. 9, pp. 552–557.
Umesha, S. and Avinash, P., Multiplex PCR for simultaneous identification of Ralstonia solanacearum and Xanthomonas perforans, 3 Biotech, 2014, vol. 5, pp. 245–252.
Remenant, B., Goutaland, B.C., Guidot, A., Cellier, G., Wicker, E., Allen, C., Fegan, M., Pruvost, O., Elbaz, M., Calteau, A., Salvignol, G., Mornico, D., Mangenot, S., Barbe, V., Médigue, C., et al., Genomes of three tomato pathogens within the Ralstonia solanacearum species complex reveal significant evolutionary divergence, BMC Genomics, 2010, vol. 11: 379.
Ramesh, R. and Phadke, G.S., Rhizosphere and endophytic bacteria for the suppression of eggplant wilt caused by Ralstonia solanacearum, Crop Prot., 2008, vol. 72, pp. 56–61.
Shanker, A.K., Djanaguiraman, M., Sudhagar, R., Chandrashekar, C., and Pathmanabhan, N., Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L.) R. Wilczek. cv. CO 4) roots, Plant Sci., 2004, vol. 166, pp. 1035–1043.
Chen, Y.P., Xing, L.P., Wu, G.J., Wang, H.Z., Wang, X.E., Cao, A.Z., and Chen, P.D., Plastidial glutathione reductase from Haynaldia villosa is an enhancer of powdery mildew resistance in wheat (Triticum aestivum), Plant Cell Physiol., 2007, vol. 48, pp. 1702–1712.
Chew, O., Whelan, J., and Millar, A.H., Molecular definition of the ascorbate–glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants, J. Biol. Chem., 2003, vol. 278, pp. 46 869–46 877.
Lin, K.H., Lo, H.F., Lin, C.H., and Chan, M.T., Cloning and expression analysis of ascorbate peroxidase gene from eggplant under flooding stress, Bot. Stud., 2007, vol. 48, pp. 25–34.
D'Arcy-Lameta, A., Ferrari-Iliou, R., Contour-Ansel, D., Pham-Thi, A., and Zuily-Fodil, Y., Isolation and characterization of four ascorbate peroxidase cDNAs responsive to water deficit in cowpea leaves, Ann. Bot., 2006, vol. 97, pp. 133–140.
Contour-Ansel, D., Torres-Franklin, L.M., de Carvalho, M.H.C., D’Arcy-Lameta, A., and Zuily-Fodil, Y., Glutathione reductase in leaves of cowpea: cloning of two cDNAs, expression and enzymatic activity under progressive drought stress, desiccation and abscisic acid treatment, Ann. Bot., 2006, vol. 98, pp. 1279–1287.
Kavitha, R. and Umesha, S., Regulation of defenserelated enzymes associated with bacterial spot resistance in tomato, Phytoparasitica, 2008, vol. 36, no. 2, pp. 144–159.
Mandal, S., Mitra, A., and Mallick, N., Biochemical characterization of oxidative burst during interaction between Solanum lycopersicon and Fusarium oxysporum f. sp. lycopersici, Physiol. Mol. Plant Pathol., 2008, vol. 72, pp. 56–61.
Trans-Kersten, J., Huang, H., and Allen, C., Ralstonia solanacearum needs motility for invasive virulence on tomato, J. Bacteriol., 2001, vol. 183, pp. 3597–3605.
Kelman, A., The relationship of pathogenicity of Pseudomonas solanacearum to colony appearance on a tetrazolium medium, Phytopathology, 1954, vol. 44, pp. 639–695.
Prakasha, A., Umesha, S., Raghava, S., and Marahel, S., Discrimination of Ralstonia solanacearum isolates by genetic signatures produced by single-strand conformation polymorphism and low-stringency single specific primer PCR analysis, Afr. J. Microbiol. Res., 2016, vol. 10, no. 30, pp. 1128–1139.
Milling, A., Babujee, L., and Allen, C., Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants, PLoS One, 2011, vol. 6, p. e15853. doi 10.1371/journal. pone.0015853
Hossain, M.A., Nakano, Y., and Asada, K., Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide, Plant Cell Physiol., 1984, vol. 25, pp. 385–395.
Feng, H., Wang, X., Zhang, Q., Fu, Y., Feng, C., Bing, W., Huang, L., and Kang, Z., Monodehydroascorbate reductase gene, regulated by the wheat PN-2013 miRNA, contributes to adult wheat plant resistance to stripe rust through ROS metabolism, Biochim. Biophys. Acta, 2014, vol. 1839, no. 1, pp. 1–12.
Kiirika, L.M., Stahl, F., and Wydra, K., Phenotypic and molecular characterization of resistance induction by single and combined application of chitosan and silicon in tomato against Ralstonia solanacearum, Physiol. Mol. Plant Pathol., 2013, vol. 81, pp. 1–12.
Mehdy, M.C., Active oxygen species in plant defense against pathogens, Plant Physiol., 1994, vol. 105, pp. 467–472.
Bao, G., Bi, Y., Li, Y., Kou, Z., Hu, L., Ge, Y., Wang, Y., and Wang, D., Overproduction of reactive oxygen species involved in the pathogenicity of Fusarium in potato tubers, Physiol. Mol. Plant Pathol., 2014, vol. 86, pp. 35–42.
Chen, S., Zimel, L., Cui, J., Ding, J., Xia, X., Liu, D., and Yu, J., Alleviation of chilling-induced oxidative damage by salicylic acid pretreatment and related gene expression in eggplant seedlings, Plant Growth Regul., 2011, vol. 65, pp. 101–108.
Radwan, D.E.M., Fayez, K.A., Mahmoud, S.Y., Hamad, A., and Lu, G., Modifications of antioxidant activity and protein composition of bean leaf due to Bean yellow mosaic virus infection and salicylic acid treatments, Acta Physiol. Plant., 2010, vol. 32, no. 5, pp. 891–904.
Lotfi, R., Gharavi-Kouchebagh, P., and Khoshvaghti, H., Biochemical and physiological responses of Brassica napus plants to humic acid under water stress, Russ. J. Plant Physiol., vol. 62, no. 4, pp. 480–486.
Prasath, D., El-Sharkawy, I., Tiwary, K.S., Jayasankar, S., and Sherif, S., Cloning and characterization of PR5 gene from Curcuma amada and Zingiber officinale in response to Ralstonia solanacearum infection, Plant Cell Rep., 2011, vol. 30, no. 10, pp. 1799–1809.
Prakasha, A. and Umesha, S., Biochemical and molecular variations of guaiacol peroxidase and total phenols in bacterial wilt pathogenesis of Solanum melongena, Biochem. Anal. Biochem., 2016, vol. 5, p. 292. doi 10.4172/ 2161-1009.1000292
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Avinash, P., Umesha, S. & Marahel, S. Role of hydrogen peroxide and ascorbate-glutathione pathway in host resistance to bacterial wilt of eggplant. Russ J Plant Physiol 64, 375–385 (2017). https://doi.org/10.1134/S1021443717030049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443717030049