-
PDF
- Split View
-
Views
-
Cite
Cite
Keith T. Jones, Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age, Human Reproduction Update, Volume 14, Issue 2, March/April 2008, Pages 143–158, https://doi.org/10.1093/humupd/dmm043
Close - Share Icon Share
Abstract
Mammalian oocytes begin meiosis in the fetal ovary, but only complete it when fertilized in the adult reproductive tract. This review examines the cell biology of this protracted process: from entry of primordial germ cells into meiosis to conception. The defining feature of meiosis is two consecutive cell divisions (meiosis I and II) and two cell cycle arrests: at the germinal vesicle (GV), dictyate stage of prophase I and at metaphase II. These arrests are spanned by three key events, the focus of this review: (i) passage from mitosis to GV arrest during fetal life, regulated by retinoic acid; (ii) passage through meiosis I and (iii) completion of meiosis II following fertilization, both meiotic divisions being regulated by cyclin-dependent kinase (CDK1) activity. Meiosis I in human oocytes is associated with an age-related high rate of chromosomal mis-segregation, such as trisomy 21 (Down’s syndrome), resulting in aneuploid conceptuses. Although aneuploidy is likely to be multifactorial, oocytes from older women may be predisposed to be becoming aneuploid as a consequence of an age-long decline in the cohesive ties holding chromosomes together. Such loss goes undetected by the oocyte during meiosis I either because its ability to respond and block division also deteriorates with age, or as a consequence of being inherently unable to respond to the types of segregation defects induced by cohesion loss.
Introduction
The main objective of this review is to provide an account of the events in the life of an oocyte, from its creation in the fetal ovary to eventual fusion with a spermatozoon following ovulation. The review aims to focus on the etiology of aneuploidy, given this process has such important clinical implications.
Defining meiosis
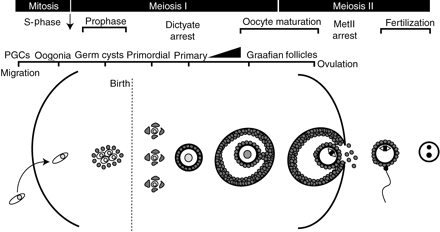
The defining feature of meiosis, in females as well as males alike, is that chromosome replication in S-phase is followed by two consecutive cell divisions to produce haploid gametes (see life cycle of oocyte in Fig. 1). The replicated pairs of chromosomes, known as homologs, recombine allowing exchange of genetic information between sister chromatids of originally paternally and maternally-derived homologs before the first meiotic division (Coop and Przeworski, 2007). Paradoxically, the large numbers of spermatozoa produced in humans are aneuploid at a rate of 1–2%, which is at least 10-fold lower than that calculated for oocytes (Hassold and Hunt, 2001; Pacchierotti et al., 2007) whose number is most probably limited to those established in fetal life. The creation of oocytes in adult life from hemopoietic stem cells appears possible (Johnson et al., 2004, 2005), however, they have been reported not to contribute to any of the cohort of mature ovulated oocytes (Eggan et al., 2006); therefore their function remains enigmatic and is not discussed further. Other reviews have concentrated on defining the incidence and exact nature of aneuploidy in human oocytes (Rosenbusch, 2004; Delhanty, 2005; Pellestor et al., 2005, 2006) and these aspects are therefore not discussed in detail here.
The life of a mammalian oocyte
The figures depicts the life of an oocyte beginning with its inception from oogonia following PGC colonization of the ovary (left) to pronucleus formation following fertilization (right), which marks the completion of meiosis and entry into the first embryonic cell cycle. See text for details
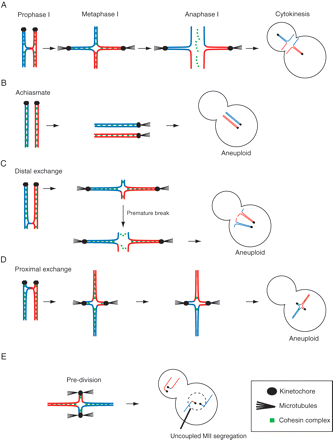
Abnormalities which result in numerical changes to the 46+XY chromosome number in humans are common and may be caused by non-disjunction of chromosomes during either of the two meiotic divisions or by premature separation of sister chromatids (so called pre-division) (Fig. 2); although their relative contribution to human aneuploidy is still debated (Wolstenholme and Angell, 2000; Rosenbusch, 2004; Pellestor et al., 2005). It is however likely that pre-division is associated more with the age-related incidence of aneuploidy (Angell, 1991; Pellestor et al., 2003; Vialard et al., 2006, 2007).
Aneuploidy in oocytes during meiosis I
A homolog pair, aligned and under tension on a metaphase I spindle: (A, C, D and E) these pairs have a single crossover event, i.e. located interstitial (A); distal (C) or proximal (D and E) in relation to the kinetochore (sister kinetochores are drawn as one for simplicity). (B) Homologs are achiasmate and so segregate independently. (E) Sister chromatids segregate during meiosis I (pre-division) due to both kinetochores of the sisters being attached to poles. In (A) the homologs segregate reductionally; in (D) the proximal exchange has prevented homolog segregation; and in (C) the pair has undergone premature separation due to the weak association of homologs
Aneuploidy and reproduction
Aneuploidy is a major obstacle in achieving reproductive success. About 20% of all human oocytes are regarded as being aneuploid; although this figure can vary widely, from ∼10% to as high as ∼40–60% (Kuliev et al., 2005; Rosenbusch and Schneider, 2006; Pacchierotti et al., 2007), and is thought to rise with increasing maternal age. It is important to consider the difficulty in getting a true reflection of aneuploidy rates in the general population, and how this may change with maternal age, given the data available are from a very selective population, failed-to-fertilize oocytes from female patients undergoing IVF (Pacchierotti et al., 2007). The vast majority of embryos formed from aneuploid oocytes are non-viable and are lost at some point before term. Trisomy 21 (Down syndrome, DS) is one of the few autosomal aneuploidies to produce viable embryos, but even here it is estimated that 80% of conceptuses are spontaneously aborted (Hassold and Jacobs, 1984). The increased use of various types of antenatal screening has been shown to detect and reduce the live DS birth rate in a number of countries despite increasing trends for older women to have children (Lai et al., 2002; Egan et al., 2004; Khoshnood et al., 2004a, b; Sipek et al., 2004; Jou et al., 2005; O’Leary et al., 2006; Coory et al., 2007), although in some reports the increased detection by screening appears to have been offset by the increased age-related incidence (Bell et al., 2003).
The success of antenatal screening and the overall incidence of DS in live births, ∼1:1000 (Morris et al., 2002; Egan et al., 2004), belies the large spectrum of clinical symptoms associated with the syndrome that have major influence on the health and welfare of affected individuals, their carers and on health service provision. Furthermore, with the increasing global first world trend to have children in later life coupled to the increased incidence of DS and many aneuploidies with maternal age (Delhanty, 2005; Pellestor et al., 2005; Sherman et al., 2005), it is likely to remain an important health issue over the next few decades.
The ‘two-hit’ hypothesis of aneuploidy
Current thinking on the derivation of aneuploidy in oocytes is dominated by the two-hit hypothesis (Lamb et al., 1996; Warren and Gorringe, 2006). These two ‘hits’ both need to occur in order to produce mis-segregated chromosomes. The first hit is a non-recombined or poorly recombined homolog pair (Lamb et al., 1996, 1997), although the derivation of this is discussed later. The second hit is an inability of the oocyte to detect and respond to this recombination failure, and in so doing allow chromosomes to mis-segregate during their meiotic division (Hawley et al., 1994). However, this second hit could in fact be the accumulation of various influences with maternal age, and these together, when summated, constitute the second hit.
It is important to consider the time interval between these two events; the first hit happens in fetal life during recombination, whereas the second hit is in the adult, in a periovulatory period during either the first or second meiotic divisions (MI and MetII, respectively). The long time interval allows the rate of aneuploidy to be affected by maternal age; theoretically by exposure to extrinsic, environmental insults (e.g. smoking; health; pollutants, etc…) which would act to accelerate the intrinsic process of aging accrued through cellular damage, e.g. by reactive oxygen species (Eichenlaub-Ritter et al., 2004). One attractive idea therefore is that such accrued damage in some way makes oocytes less able to sense recombination errors that arose in prophase during fetal life. However, it is also possible that oocytes remain poor at this detection throughout life, and instead it is the ties which hold chromosomes together that simply get weaker with age, damaged by aging and unable to be properly repaired (Wolstenholme and Angell, 2000).
Passage from mitosis to GV stage arrest
From PGC to oocyte
The tenet of the ‘two-hit’ model is that fetal recombination errors constitute the first hit. Therefore it is important to discuss how primordial germ cells (PGCs) undergo entry into meiosis, and this will be done in mice where detail is most known. PGCs appear not to enter meiosis completely synchronously and such findings may have implications for the quality of oocytes produced as is discussed later.
In mouse fetal life at 7 days post-coitum (days pc), ∼45 PGCs (Lawson and Hage, 1994) migrate from the base of the allantois to a structure that becomes the genital ridge (McLaren, 2003; Wilhelm et al., 2007). Here the now 3000 PGCs, termed gonocytes, continue to divide mitotically until 13.5 days pc (Monk and McLaren, 1981; McLaren, 2000), when in females they commit to enter meiosis. Retinoic acid (RA) produced by the associated mesenopheros appears to be critical in inducing entry into meiosis of female gonocytes (oogonia) (Bowles et al., 2006; Koubova et al., 2006); however, PGCs on entering the genital ridge are initially unable to respond to the RA signal because of the action of the cyp26b1 gene, which produces a RA-metabolizing P450 cytochrome enzyme (Bowles et al., 2006). CYP26B1 expression falls off in oogonia, such that by 12.5 days pc its expression is male-specific. Loss of cyp26b1 expression in females between 10–12.5 days pc, allows RA to stimulate oogonia commitment to meiosis. One important target gene for RA is Stimulated by Retinoic Acid gene 8 (Stra8), since in Stra8 knockouts, oogonia fail to undergo any of the critical events of entry into the pre-meiotic S-phase which eventually lead to recombination (Baltus et al., 2006). An important feature of RA-induced entry into meiosis is the source of the RA, which is located in the associated mesenopheros (Bowles et al., 2006), this would form a gradient of RA within the developing gonad. Such a gradient probably underlies the observed spatial wave, anterior to posterior, of meiosis entry in mouse fetal ovaries, judged by the switch off of germ cell markers such as Oct-4, and the switch on of meiotic markers such as Stra8, Dmc1 and Sycp3 (Menke et al., 2003; Bullejos and Koopman, 2004). A similar wave of meiosis entry probably also occurs in the human ovary, judged by the finding that oogonia committing to leptotene first at around 9 week pc are closest to the mesonephros, although oogenesis appears less strictly timed (Bendsen et al., 2006).
Meiotic prophase: from leptotene to dictyate-arrest
The process of meiotic recombination in oocytes begins on 13–14 days pc in mouse (Speed, 1982) and in the 10–11th week of gestation in humans (Kurilo, 1981; Garcia et al., 1987). During leptotene, homolog pairs align and then synapse with the formation of the synaptonemal complex during zygotene (Zickler and Kleckner, 1998; Walker and Hawley, 2000; Page and Hawley, 2004). The synaptonemal complex marks the site of crossovers and probably provides the structural framework in which to execute double strand breaks. Double strand breaks are initiated by the topoisomerase-like enzyme Spo11 (Keeney and Neale, 2006), whose loss in mouse blocks this process leading to sterile animals (Baudat et al., 2000; Romanienko and Camerini-Otero, 2000) and whose mutation may be responsible for some infertility in humans (Mori et al., 2006). The proteins involved in synapsis, crossover exchange and synaptonemal complex formation are partly known in mammals, and such identification is often aided by having yeast homologs. Invariably, knockout of these genes produces sterile mice (Pittman et al., 1998; Yuan et al., 2002; Li and Schimenti, 2007). The creation of double strand breaks requires oocytes to have a mechanism for a meiosis-specific DNA break repair which uses as template the homolog rather than the sister strand (Kleckner, 2006). It also necessitates a surveillance mechanism which monitors for unrepaired double strand breaks at a time when recombination should have been completed. Defects in genes (eg dmc1 and Msh5) involved in the processing of meiotic double strand breaks produces a severe loss of oocytes shortly before and after birth (Di Giacomo et al., 2005; Morelli and Cohen, 2005).
The synaptonemal complex dissolves leaving the physical manifestations at points where crossover has occurred, chiasmata, visible in diplotene. The oocyte then arrests with decondensed chromatin at this stage, often referred to as dictyate-stage arrest. In mouse, most oocytes have reached the dictyate stage by 20 days pc (Speed, 1982), while it is less synchronous in humans with dictyate oocytes observed from 14–15 weeks pc onwards (Kurilo, 1981; Garcia et al., 1987). In mouse, entry into meiosis is not synchronous: oogonia first enter meiosis at ∼13.5 days pc and this process continues in a wave which is nearly complete about 3 days later (Peters, 1970; McLaren, 2000). Parturition is at ∼20 dcp and oocytes have completed prophase by Day 1 post-partum (Speed, 1982). In humans, the passage of oogonia into meiosis appears far more spread during gestation. Here leptotene stage oocytes can first be detected at ∼10 weeks and these oocytes appear to take around 5 weeks to reach the dictyate stage (Kurilo, 1981; Garcia et al., 1987). However, oocytes continue to be observed at all stage of meiosis throughout gestation.
Entry into meiotic prophase and aneuploidy
It is important to note that crossover happens for each homolog pair, even the X and Y chromosomes in spermatogenesis, which pair through their pseudoautosomal regions (Graves et al., 1998; Blaschke and Rappold, 2006). The crossovers, in conjunction with sister chromatid cohesion, allow homologs to stay paired when pulling forces from microtubules are generated during MI (Fig. 2A). A failure to recombine means that homologs cannot remain associated together during MI, they therefore segregate randomly (Fig. 2B). The generation of achiasmate homologs is likely to account for around 50% of all DS conceptuses whose origin is in a non-disjunction during MI (Lamb et al., 1997). If the exchange is near the end of the chromosomes it follows that during MI it would only be the small distal exchange regions that could generate the counter-tension from microtubule pulling forces. A priori, exchange at sites near the telomeres of chromosomes should generate recombined homologs which are sensitive to non-disjunction (Fig. 2C). This has indeed been borne out by studies in yeast artificial chromosomes (Ross et al., 1996) and flies (Koehler et al., 1996). Exchange in a pericentromeric location also appears to generate a configuration susceptible to non-disjunction (Lamb et al., 1997; Hassold et al., 2000). Here the pericentromeric crossover may interfere with centromeric cohesion and/or kinetochore attachment to allow both homologs to move to the same pole (Fig. 2D). Or pericentromeric crossover may interfere with the essential block in MI to sister kinetochrores being attached to both spindle poles, which therefore allows sister chromatids to be separated in MI, rather than during metaphase II, MetII (so called ‘pre-division’; Fig. 2E).
In the two-hit model, susceptible patterns of recombination forming the first hit can be generated stochastically during prophase because it is the second hit which is believed to be age related. However, in mouse it has long been observed that chiasmata frequency in oocytes decreases with maternal age (Henderson and Edwards, 1968; Luthardt et al., 1973; Polani and Jagiello, 1976). Since the recombination pattern of homologs is established in fetal life, Henderson and Edwards (1968), who were the first to identify this phenomenon, suggested that such observations were explained by fetal oocytes entering meiosis in an order which mirrored their exit in the adult. Oocytes first to enter meiosis are those that mature first in the adult so resembling a production line. The further proposition being that the late entry of oocytes into meiosis negatively affects their ability to recombine.
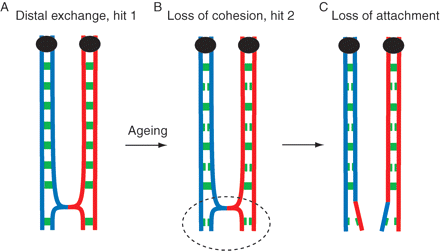
Because the molecular details of sister attachment is now known, it is probable that the visible loss of chiasmata observed previously is likely due to an age-associated loss of sister chromatid cohesion (Fig. 3). Therefore no production line has to be invoked to account for the age-related decrease in the incidence of chiasmata. However, it remains an interesting unresolved question as to whether the oocytes first to commit to meiosis in the fetus are the first to be ovulated in the adult. It seems clear that oocytes do enter meiosis in a wave (as detailed above), however, is that wave similarly mirrored in ovulations during adult life? Experiments designed directly to demonstrate the phenomenon of ‘first in–first out’ have not been extensive and unfortunately remain equivocal (Polani and Crolla, 1991; Hirshfield, 1992; Meredith and Doolin, 1997). An in vitro labeling of pre-meiotic S-phase mouse oogonia by radiolabeled nucleotide, followed by transplantation, demonstrated that early labeled oogonia go on to mature early (Polani and Crolla, 1991). Complementary findings in the rat using an in vivo method of delivery demonstrated that late labeled oogonia were not observed in growing follicles of adult rats and instead formed a pool of the most quiescent primordial follicles (Hirshfield, 1992). However, a similar approach again in rat failed to verify a strict production line with early entry oocytes being found in growing follicles at all ages (Meredith and Doolin, 1997), but it did show late entry late entry oocytes were more quiescent than those with early entry.
Loss of cohesin with age resolves chiasmata
A single crossover event has happened in fetal life for this homolog pair (A). With age the cohesive ties holding homologs weaken (B). This distal exchange has been affected by the loss of distal cohesin such that the homolog pair are achiasmate and will segregate randomly at meiosis I
For the production line hypothesis to be important here, in the derivation of aneuploidy, then one would have to assume the process of meiosis is hindered somehow by late entry. Interestingly, it is of note that late entry oogonia would have likely undergone more replications than the early oogonia. During this replication period one would anticipate cells maintain high telomerase activity; the enzyme responsible for maintaining adequate telomere length to the end of chromosomes. Indeed, telomerase activity is very high in the fetal ovary (Wright et al., 1996). Defective telomeres, however, in yeasts, reduce recombination rates during meiosis (Cooper et al., 1998; Nimmo et al., 1998; Rockmill and Roeder, 1998) and it has therefore been suggested that telomere shortening in late entry oogonia may be a determinant in aneuploidy (Keefe et al., 2006, 2007). It remains to be established if telomere shortening is observed in oocytes from older women.
The cohesin complex
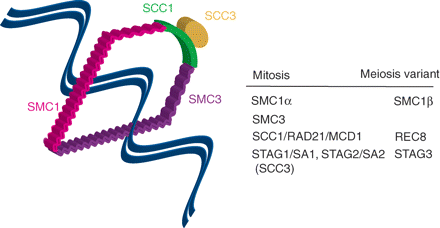
It is important to consider the ties which hold chromosomes together. These ties if perturbed could potentially account for mis-segregation. As it will be discussed, they are set up in fetal life during prophase and as such offer a long-standing target for the generation of aneuploidy by non-disjunction. First, to understand the process in meiosis we have to examine how sisters are held together during mitosis. One important feature of mitosis is that to ensure fidelity in sharing genetic information, chromosomes are first aligned on a metaphase spindle and only once alignment is achieved does anaphase onset occur (Mitchison and Salmon, 2001; Tanaka, 2002). Newly replicated sister chromatids are held together in S-phase onwards by cohesin complexes (Fig. 4), which are believed to form ring-like structures embracing both sisters (Gruber et al., 2003; Uhlmann, 2004; Nasmyth and Haering, 2005). A heterodimeric pair SMC1 and SMC3, members from the structural maintenance of chromosomes (SMC) family, and SCC1/RAD21/MCD1 and SCC3, which has two vertebrate isoforms STAG1/SA1 and STAG2/SA2, constitute the cohesin complex. The SMC1–SMC3 heterodimer joins in a head-to-head and tail-to-tail configuration, with their tails forming a hinge-like structure (Hirano, 2006). At their head they associate with SCC1 and SCC3, proteins which are modified when cohesin’s hold on sisters needs be released during the mitotic division.
The cohesin complex
Sister chromatids are held together by the cohesin complex, which embraces them in a ring-like structure. The components of the cohesin complex are labeled and their meiotic counterparts tabulated. Note that at anaphase-onset separase proteolytically cleaves Sister chromatin cohesion protein 1 (SCC1), allowing sisters to separate under pulling forces from spindle microtubules. SMC, structural maintenance of chromosomes protein
In meiosis some of the mitotic components of the cohesin complex are replaced (Fig. 4). Therefore SCC1 is largely replaced by REC8 (Klein et al., 1999; Parisi et al., 1999; Watanabe and Nurse, 1999); SA1/SA2 by STAG3 (Pezzi et al., 2000; Prieto et al., 2001) and SMC1 (now better named SMC1α) by SMC1β (Revenkova et al., 2001). Having a meiotic variant is likely to confer some specialized meiotic function: e.g. in fission yeast REC8 can rescue mitosis loss of SCC1, but SCC1 cannot rescue meiotic loss of REC8 (Watanabe and Nurse, 1999); similarly, mammalian REC8 can bind to SMC1β but not SMC1α (Lee et al., 2003). Loss of REC8 in mouse leads to homolog pairing but no homolog synapsis (Xu et al., 2005). One possible further meiotic function for REC8 observed in yeasts and Arabidopsis is in the establishment and maintenance of monopolar orientation of the MI sister kinetochores (Chelysheva et al., 2005; Yokobayashi and Watanabe, 2005). This is necessary only in MI because sister kinetochores act as a functional unit binding to the same pole, a process in budding yeast known to be dependent on the Aurora B kinase (Monje-Casas et al., 2007) and the Monopolin complex (Toth et al., 2000; Rabitsch et al., 2001), whose mammalian homologs intriguingly remain unknown.
Passage through meiosis I
Loss of oocytes during early life
Oocytes from newborn animals are often connected by cytoplasmic bridges in aggregates known as ‘cysts’ (Pepling and Spradling, 1998). These cysts rapidly brake down in the days following birth and oocytes individualize (Pepling and Spradling, 2001); this is discussed in an account elsewhere (Pepling, 2006). Following birth, oocyte numbers drop substantially and such loss of oocytes may be similar to the so-called pachytene, or meiotic recombination, checkpoint in budding yeasts (Roeder and Bailis, 2000). The oocyte within primordial and primary follicles does have the capacity to detect DNA damage through the p53-like gene, TA-p63α (Suh et al., 2006), a process which intuitively should be active to get rid of any DNA-damaged oocytes during their period of dictyate arrest. However, it is also clear that oocytes also have a DNA damage independent mechanism of cell death (Di Giacomo et al., 2005).
Signalling between oocyte and follicular cells
Outside the scope of this review is the cyclical recruitment of ovarian follicles from the non-growing pool (McGee and Hsueh, 2000; Webb et al., 2003; Knox, 2005; Visser and Themmen, 2005). Important in the health of the oocyte during this period of arrest is bi-directional communication between the oocyte and the follicular granulosa cells (Eppig, 2001). Various factors are likely provided to the oocyte by the follicular somatic cells which support growth, and here interestingly the cumulus-derived sterol FF-MAS may act to suppress pre-division (Cukurcam et al., 2007). Conversely, oocyte-derived members of the transforming growth factor (TGF)β superfamily act as paracrine factors to influence follicle development (Drummond, 2005), such as Growth Differentiation Factor-9 whose loss blocks growth of primary follicles (Dong et al., 1996). The close relationship between oocyte and surrounding granulosa cells is highlighted by the fact that oocytes are deficient in their ability to generate ATP through the glycolytic pathway; instead they secrete fibroblast growth factor and bone morphogenetic factor 15, a member of the TGFβ superfamily, to stimulate glycolytic activity in adjoining granulosa cells and in so doing provide them with nutrients (Sugiura et al., 2007). Oocyte-derived TGFβ paracrine factors are also involved in stimulating granulosa cell growth, preventing apoptosis and play a role in the process of cumulus cell expansion which is associated with oocyte maturation (Hussein et al., 2005; Gilchrist et al., 2006; Dragovic et al., 2007; Hutt and Albertini, 2007).
From GV arrest to polar body extrusion
Having undergone S-phase during early fetal life as discussed earlier oocytes remain arrested within growing follicles. The transition from the dictyate stage into diakinesis, which occurs physiologically within large antral follicles, forms part of a process termed ‘oocyte maturation’ or ‘meiotic maturation’, which encompasses other cellular events that prepare the oocyte for fertilization, such as a maturation of the cortical granules responsible for the block to polyspermy (Ducibella et al., 1988; Abbott et al., 1999). Associated loss of the nuclear envelope (called ‘germinal vesicle’, GV, in oocytes) gives the start of the process the name of germinal besicle breakdown (GVB or GVBD). Nuclear maturation is the name given to the events controlling the actual cell cycle progression leading to eventual arrest at MetII, whereas cytoplasmic maturation refers to events and processes that are not chromatin based, such as the aforementioned cortical granule maturation. Here the review will concentrate only on nuclear maturation, which is driven by rising cyclin-dependent kinase (CDK1) activity.
Oocyte maturation finishes with a small first polar body (PB1) being extruded. This unequal cell division results from a cortical migration of the spindle dependent on actin microfilaments (Verlhac et al., 2000), and the actin-binding protein formin-2 (Leader et al., 2002; Dumont et al., 2007). As the spindle nears the oolemma, a region above the spindle accumulates actin, myosin, Par6 and the small GTPases Rho, Rac and Cdc42 (Vinot et al., 2004; Na and Zernicka-Goetz, 2006; Deng et al., 2007; Halet and Carroll, 2007), which are essential for polarity in a number of systems. This cortical actin cap is therefore associated with the spindle pole nearest the plasma membrane, and with its associated set of separated homologs is cleaved from the oocyte by a myosin ring constriction. Presumably, the purpose of an unequal cell division is to ensure retention of maternal RNA and protein useful for MetII and early embryogenesis within the oocyte, as well as providing a too small a target for any fertilizing sperm that may fuse with the polar body rather than the oocyte proper.
cAMP in maintaining GV arrest
Once follicles become fully grown, continued dictyate arrest requires high protein kinase A (PKA) activity inside the oocyte. Therefore, removal from the ovary necessitates addition of cyclic AMP (cAMP) analogs or phosphodiesterase inhibitors to the culture media to maintain arrest (Eppig, 1989; Tornell et al., 1991; Conti et al., 1998; Dekel, 2005; Mehlmann, 2005). In vivo a G-protein coupled receptor on the plasma membrane of the oocyte is a constitutively-active receptor providing adequate cAMP to maintain GV arrest (Mehlmann et al., 2002, 2004). Whether the periovulatory surge in LH switches off this receptor or up-regulates phosphodiesterases in order to lower PKA activity remains to be established (Mehlmann et al., 2006). Also still under investigation are the intermediaries which provide the necessary signals from the rise in LH to oocyte, since oocytes lack LH receptors. Possibly involved is a cumulus cell-derived sterol FF-MAS (Byskov et al., 1995, 2002), but the physiological relevance of FF-MAS to LH-induced meiotic resumption is now questioned and other mechanisms likely (Tsafriri et al., 2005; Conti et al., 2006). Despite all these uncertainties, it is generally agreed that further progression through MI is mediated by a drop in intra-oocyte cAMP.
CDK1 and GVB
Critical to maintenance of GV arrest is low CDK1 activity (a.k.a maturation or M-phase promoting factor; cdc2; p34cdc2), a kinase implicated in dissolution of the nuclear envelope and condensation of chromatin (Doree and Hunt, 2002; Jones, 2004): two events associated with entry into the first meiotic division. Key to maintaining low CDK1 is regulation of the kinase and phosphatase responsible for phosphorylating/dephosphorylating CDK1, respectively (Fig. 5). Thus during GV arrest the activity of wee1B, the kinase which negatively affects CDK1 activity through phosphorylation is high, while cdc25B, the phosphatase responsible for activating CDK1 is low; probably by direct phosphorylation through PKA (Duckworth et al., 2002; Lincoln et al., 2002; Han et al., 2005). The ability of PKA to negatively affect CDK1 activity, and maintain it in a so-called pre-MPF state, would be the physiological basis of the need to maintain a high cAMP drive in oocytes for GV arrest. Interestingly, although multiple members of the wee1 and cdc25 family exist, specific isoforms are responsible for regulating CDK1 in mammalian oocytes (Lincoln et al., 2002; Han et al., 2005). For example, oocytes from cdc25b knockout mice lack the ability to undergo GVB, even though they contain cdc25a and cdc25c (Lincoln et al., 2002).
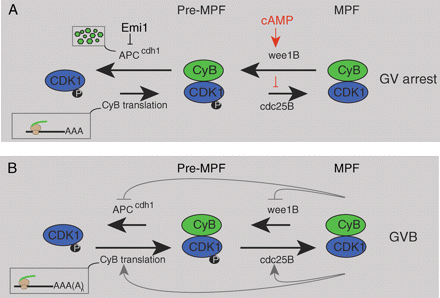
Germinal vesicle stage arrest
(A) Oocytes from mature follicles are prevented from undergoing GVB by high intra-oocyte cAMP levels. cAMP acts via protein kinase A to keep wee1B active and cdc25B inactive. In so doing, maturation-promoting factor (MPF) is maintained in a phosphorylated and inactive state (Pre-MPF). A reduction in cAMP is believed to be the physiological trigger for inducing GVB. Also during GV arrest levels of cyclin B1 (CyB), the regulatory partner of CDK1, are controlled through degradation mediated by the anaphase-promoting factor bound to its co-activator cdh1 (APCcdh1), which is itself regulated by Emi1. During GV arrest cyclin B1 levels must be maintained by some mRNA translation to nullify its continual degradation. (B) Loss of cAMP at GVB allows MPF activity to rise. This negatively affects wee1B activity but stimulates that of cdc25B. Rising MPF may also switch on more cyclin B1 translation and will act eventually to switch off APCcdh1 activity (although this latter event does not happen until several hours after GVB). See text for further details
Although in vivo release from GV arrest is likely mediated through regulation of CDK1, cyclin B1 regulation also appears important for maintenance of GV arrest. This is because it is possible to raise CDK1 activity by overexpressing cyclin B1, the regulatory partner of CDK1, and in so doing induce GVB even in the presence of high cAMP (Ledan et al., 2001; Marangos and Carroll, 2004; Reis et al., 2006). Thus there is turnover of cyclin B1 in the GV-arrested oocytes to prevent precocious GVB (Reis et al., 2006). This is achieved through the anaphase-promoting complex/cyclosome (APC), a large multisubunit complex which acts as an E3 ubiquitin ligase, able to bind specific substrates such as cyclin B1 and earmark them for proteolysis through the 26S proteasome by a process of polyubiquitination (Peters, 2006). The APC is active in GV-arrested mouse oocytes, through association with one of its two key activators, cdh1, and this APCcdh1 activity is needed to maintain oocytes in GV arrest (Reis et al., 2006).
APCcdh1, the regulator of cyclin B1 levels in oocytes, is itself regulated during GV arrest by association with its inhibitor Emi1. Emi1 was first described as a G2-phase cell cycle regulator of APC—acting to prevent precocious APC activity before mitosis (Reimann et al., 2001). Therefore if Emi1 is knocked-down in oocytes, it increases APCcdh1 activity and makes oocytes refractory for GVB, presumably by lowering cyclin B1 (Marangos et al., 2007). Conversely, overexpressing Emi1 leads to GVB, presumably by inhibition of APCcdh1 allowing cyclin B1 levels to accumulate.
Oocytes from preantral follicle of an adult mouse or oocytes from young mice (Day 15 or younger) fail to undergo maturation in culture (Erickson and Sorensen, 1974; Sorensen and Wassarman, 1976). This is most likely due to insufficient levels or insufficient interaction of CDK1 and cyclin B1 (de Vant’ery et al., 1996, 1997; Mitra and Schultz, 1996; Kanatsu-Shinohara et al., 2000). Indeed, overexpression of both CDK1 and cyclin B1 is needed to induce GVB in oocytes from young mice (de Vantery et al., 1997). It remains a challenge to refine in vitro culture systems for human oocytes derived from preantral stages of follicle growth, in order to deliver oocytes that are capable of undergoing in vitro maturation and eventual fertilization (Picton et al., 2003). Especially so, since it is during oocyte growth that various mRNAs are made and stored, disruption of this process severely affecting later oocyte maturation and fertilization (Yu et al., 2004), and is also when imprints are laid down for proper control of gene expression (Obata and Kono, 2002).
Interestingly, frog eggs contain an activator of CDK1 which is not cyclin B (Ferby et al., 1999). This protein, called RINGO (rapid inducer of G2/M progression in oocytes) is able to bind CDK1 so inducing GVB and its loss from frog oocytes blocks this transition. Its role in the process of GVB in mammalian oocytes is little explored. Frog RINGO can induce GVB in mouse and pig oocytes but blocks them in MI (Terret et al., 2001; Kume et al., 2007), whereas pig RINGO allows oocytes to complete MI (Kume et al., 2007). This suggests that mammalian RINGO homologs may have a physiological role in GVB. Future experiments are therefore required to assess the relative contribution of cyclin B1 and RINGO in the activation of CDK1 to trigger GVB.
Cohesin loss: meiosis V mitosis
The completion of the first meiotic division, from GVB to the extrusion of the PB1 marks the period in which the second hit, important in the derivation of aneuploidy, is thought to operate. In essence, it is regarded as an age-related decline in the ability of the maturing oocyte during MI to respond to errors in non- or poorly recombined homologs. It is first worth examining how faithful segregation of chromosomes (sister chromatids) are achieved in mitosis and then examining if the same mechanisms are known to operate in oocytes during this first, reductional division. During prometaphase in mitosis, non-centromeric cohesin complexes are removed by phosphorylation rather than proteolysis: involving Aurora B and polo (Losada et al., 2002; Sumara et al., 2002; Gimenez-Abian et al., 2004). Polo-kinase directly phosphorylates SCC3 subunits (Hauf et al., 2005). However, centromeric SCC3 is protected from phosphorylation by the protein Shugoshin (Sgo) and the phosphatase PP2A until the metaphase–anaphase transition (McGuinness et al., 2005; Kitajima et al., 2006; Riedel et al., 2006). Loss of centromeric cohesin at anaphase is caused by proteolysis of its SCC1 subunit by the protease separase (Uhlmann et al., 1999, 2000; Waizenegger et al., 2000; Hauf et al., 2001), possibly aided by degradation of Sgo (Salic et al., 2004; Fu et al., 2007). Premature activity of separase is prevented through binding the protein securin; which in mammals was first described as pituitary tumour transforming gene1 (Zou et al., 1999; Waizenegger et al., 2000). Therefore when sister chromatids have fully aligned on a metaphase plate, timely separation is achieved by securin proteolysis, which allows separase to act on Scc1, a process first described in yeasts (Ciosk et al., 1998; Uhlmann et al., 1999).
In meiosis, cohesin is lost in a step-like manner, with non-centromeric cohesin removed during MI; and centromeric cohesin, which was protected by Sgo1, during MetII. Both the non-centromeric loss of cohesin in MI and the centromeric loss in MetII seem to be dependent on REC8 proteolysis by separase (Huo et al., 2006; Kudo et al., 2006; Lee et al., 2006). This therefore appears to be different to mitosis where the non-centromeric loss would be driven not by proteolysis, but by polo-kinase (Hauf et al., 2005).
Regulation of the mitotic division by the SAC
In order to understand how segregation of homologs may be regulated during MI, we first must examine how sister segregation is regulated in mitosis. At the metaphase–anaphase transition mitotic cells need to decrease their CDK1 activity (Peters, 2002, 2006; Pines, 2006). This is achieved by degradation of CDK1’s regulatory partner cyclin B1 (Jones, 2004). Loss of cyclin B1, and also securin to free separase and so cleave SCC1 (or REC8), is through APC-dependent proteolysis. The high CDK1 activity prevents cdh1 association with the APC (Zachariae et al., 1998; Jaspersen et al., 1999; Blanco et al., 2000), instead it is another WD-repeat protein cdc20 which is required for APC activity at metaphase (Peters, 2002, 2006; Pines, 2006). Before a mitotic cell wants to commit to segregating its chromosomes, APCcdc20 activity is held low by activation of the spindle assembly checkpoint (SAC) (Musacchio and Salmon, 2007). The SAC pathway is active until chromosomes are fully aligned and under tension, with sister pairs attached to opposite spindle poles, a condition called bi-orientation. There are many protein members of the SAC that were initially identified from studies in yeasts in genetic screens of cells failing to arrest in mitosis in the presence of spindle poisons that disrupted assembly of the spindle (Hoyt et al., 1991; Li and Murray, 1991; Li et al., 1993; Weiss and Winey, 1996). These include the mitotic arrest deficient gene products Mad1, Mad2 and Mad3 (BubR1 in mammalian cells) and the budding uninhibited by benzimidazole gene product Bub1, Bub2 and Bub3 as well as the protein Mps1.
Members of the SAC are thought to be able to respond to a lack of tension and/or attachment of microtubules to the kinetochore by blocking the ability of cdc20 to bind and so activate the APC (Hwang et al., 1998; Kim et al., 1998). The relative contribution of tension and attachment to activation of the SAC is much debated and difficult to ascertain because of their inter-dependence (Pinsky and Biggins, 2005). A lack of attachment consequently leads to a lack of tension; which can also weaken attachment, a necessary process in establishing correct attachment of sister kinetochores to opposite spindle poles. However, a mitotic checkpoint complex containing Mad2, BubR1 and Bub3, in addition to cdc20, appears to be the effector of the SAC and is thought to require kinetochores for its assembly (Sudakin et al., 2001; Musacchio and Salmon, 2007).
The SAC in meiosis
Attempts to examine if SAC components are absent or somehow non-functional in oocytes have in fact often reached the surprising conclusion that they are present and active. Rodent oocytes are known to contain Mad2 (Wassmann et al., 2003; Zhang et al., 2004; Homer et al., 2005a, b; Wang et al., 2007) and Bub1 (Brunet et al., 2003; Yin et al., 2006); and importantly knocking down or interfering with their function, as well as that of BubR1, accelerates passage through MI and, when examined, leads to mis-segregated homologs (Tsurumi et al., 2004; Zhang et al., 2004; Homer et al., 2005a, b; Yin et al., 2006; Wang et al., 2007). This suggests that normally Mad2, Bub1 and BubR1 all function in slowing passage through MI, and by implication constitute an active SAC.
It is important to consider what precisely are the triggers for activation of the SAC during MI. Each sister kinetochore pair must act as a functional unit to establish bi-orientation of the bivalent. It is therefore interesting that oocytes from female mice carrying a univalent X fail to show any metaphase arrest (LeMaire-Adkins et al., 1997). In these oocytes it is possible that univalents evade the SAC by bi-orientating their sister kinetochores and so undergoing pre-division (Fig. 2), as observed in oocytes lacking Sycp3, a component of the synaptonemal complex (Kouznetsova et al., 2007). However, the univalent X is reported to segregate reductionally, suggesting that tension across the univalent is not detected. Interpreting these observations and with those of the Mad2, Bub1 and BubR1 data described above, suggests that oocytes do possess a SAC-like mechanism which can halt segregation of chromosomes during MI, however, it does not necessarily monitor homolog bi-orientation. Recent work in budding yeast during MI suggest that Mad2, and by implication the SAC, may only be important in re-orientating homologs that have distal sites of crossover from the kinetochore (Lacefield and Murray, 2007). The argument being that with proximal exchange the bivalent has a more rigid geometry which predisposes it to bi-orientation. However in distal crossovers the bivalent could be considered more lithe and open to mono-orientation of sister kinetochores.
It has been suggested that an early prometaphase SAC-like mechanism, dependent on Mad2 and BubR1 but independent of kinetochores, is present in cells which can delay passage through mitosis (Meraldi et al., 2004). Such a delay could be due to an ability to bind and so sequester cdc20 (Burton and Solomon, 2007). This early mechanism is then followed by a true SAC, dependent on kinetochores, later in prometaphase (Musacchio and Salmon, 2007). It follows that loss of SAC proteins such as Mad2 and BubR1 could accelerate MI, without there being a proper kinetochore-dependent SAC in oocytes. Similarly, overexpression of SAC proteins such as Mad2, known to block oocytes at metaphase I (Homer et al. 2005a, b), would act due to an ability to bind and sequester cdc20 from the APC, rather than by imposition of a SAC. However the simplest experiment, addition of spindle poisons, to oocytes have all demonstrated directly the existence of a SAC by blocking oocytes in MI (Wassmann et al., 2003; Homer et al., 2005a, b). Clearly, whatever the detail is in the SAC-imposed arrest upon addition of spindle poisons, such a pathway is not executed when univalent homologs are present in MI.
Control of CDK1 loss during MI
One intriguing feature of MI is its extraordinary length. In cultured cells undergoing mitosis, from the time of nuclear envelope breakdown to cytokinesis is ∼20–30 min (Rieder et al., 1994; Meraldi et al., 2004), compared with the several hours from GVB until PB1 extrusion in mammalian oocytes. The extended period of meiosis is reflected in the dynamics of CDK1 activity, which rises abruptly in mitosis but gradually in meiosis, reaching a peak several hours after GVB (Choi et al., 1991; Gavin et al., 1994; Verlhac et al., 1994, 1996). Loss of CDK1 activity is an essential requirement for completion of MI, and specifically degradation of cyclin B1. Also important is degradation of securin to free separase. In mouse, loss of both cyclin B1 and securin occur synchronously in a period which terminates with PB1 extrusion and is dependent on APCcdc20 (Reis et al., 2007), as it is in mitosis. However, early in prometaphase I APCcdh1 is active, made possible by low CDK1 activity at this time, and here it degrades cdc20. This means cdc20 has to be resynthesized in order for oocytes to complete MI and suggests an unusual progression through chromosome segregation not observed with sisters in mitosis. Loss of cdh1 brings forward the period of APCcdc20 activity and consequently the period of cyclin B1 and securin degradation (Reis et al., 2007). This premature metaphase I induces high rates of non-disjunction and leads to a disruption of the integrity of the MetII spindle probably as a consequence of a chromosome crowded spindle if hyperploid. Similar reasoning may be behind the disruptions of the spindle structure that have been observed in human oocytes from older women (Rosenbusch and Schneider, 2006; Shen et al., 2006). Interestingly, the SAC had not been switched off in these cdh1-depleted oocytes (Reis et al., 2007), since spindle poisons were equally effective at blocking exit from MI whether cdh1 was present or not. Therefore the acceleration of MI generated mis-segregated homologs and the SAC was not activated. Again, this suggests the inability of the SAC to monitor homolog bi-orientation.
Loss of CDK1 activity through degradation of cyclin B1 may not be the sole mechanism for regulating CDK1 in oocytes. Free separase, generated by proteolysis of securin, can also bind CDK1 and in so doing inhibit its kinase activity (Stemmann et al., 2001, 2006); in fact, inhibition is mutual since CDK1 binding inhibits separase proteolytic activity too (Gorr et al., 2005). The ability of separase to bind CDK1 also appears essential for PB1, since PB1 extrusion is blocked when separase binding is inhibited (Gorr et al., 2006). The ability of CDK1 activity in oocytes to be regulated by both loss of cyclin B1 and separase binding warrants further investigation given this process is required for completion of MI. Future studies are therefore required to assess their inter-dependence.
The SAC or cohesion deteriorates with age?
It remains possible that SAC function could deteriorate with maternal age to account for the age-related incidence of aneuploidy. Therefore one could argue that the SAC is fully functional in oocytes from younger women, but its ability to respond to mis-alignment of homologs on the MI spindle weakens in oocytes from older females. Mad2 protein and mRNA both seem relatively unstable in MetII oocytes making such a hypothesis attractive if their instability extends to immature oocytes (Ma et al., 2005; Steuerwald et al., 2005). Interestingly, these authors hypothesize that the post-ovulatory loss of Mad2 may be responsible for the increased sister chromatid segregation defects associated with post-ovulatory aging. However, the SAC, although it can be activated by various spindle poisons, is currently thought not to be involved in maintaining the physiological arrest during MetII in mammalian oocytes, so establishing any causal link would be important (Tsurumi et al., 2004; Madgwick et al., 2006; Shoji et al., 2006; Madgwick and Jones, 2007).
Only one study has addressed if SAC components decrease in human oocytes with age. Here it was found that transcript levels of both Mad2 and Bub1 decrease with increasing maternal age (Steuerwald et al., 2001). It will be important to corroborate this study and also provide direct evidence that the SAC is indeed compromised in oocytes from older women during MI. This would address whether the reduced transcript levels affect protein levels and consequently SAC function. Interestingly, levels of hundreds of transcripts, including cell cycle genes, have been been reported to decrease with increased maternal age in mice and women (Hamatani et al., 2004; Steuerwald et al., 2007). This raises the possibility that aneuploidy may result due to falling levels of various components of the cell cycle, not just SAC members.
One possible target rather than the SAC to explain age-related aneuploidy is the cohesin complex itself. Theoretically, if functionality of the complex were to decline with age it would bring together many of the loose threads presented thus far. For example, the reduced recombination frequency, assessed by chiasmata, with age in mouse (Henderson and Edwards, 1968). This was interpreted in terms of a production line because of recombination frequency being established in fetal life. However, one would observe a similar loss in chiasmata if the cohesin ties holding homologs together were loosened during dictyate arrest to the extent of separating homologs. The fact that the cohesin complex holding homologs together is established during fetal S-phase and yet has to remain functional decades later would make it susceptible to age-related damage because it may be difficult or impossible to repair. Mutation of the meiosis-specific cohesin SMC1β makes female mice sterile due to large levels of aneuploidy (Revenkova et al., 2004). Interestingly, these mice display an age-related incidence in aneuploidy with 4-week old mice containing oocytes with bivalents only, whereas essentially no intact homologs could be observed by 4 months (Hodges et al., 2005). The ability of the aneuploidy rate to rise with maternal age suggests that during early oocyte growth and follicle maturation cohesins may deteriorate and need to be replaced. The inability of these SMC1β mutant mice to perform this replacement would then account for the maternal age effect. One would predict that oocytes in which higher than average rates of recombination exist may be in part protected against any age-related loss in sister chromatid cohesion, and indeed human studies examining recombination frequency of offspring against their mother’s age appear to bear this out (Kong et al., 2004).
Age-associated aneuploidy: environmental and hormonal factors
An age-related decline in the cohesive ties holding chromosomes together coupled to an intrinsic inability of oocytes to detect or repair the resulting error is likely to constitute the etiology of some aneuploidy. This process may be exacerbated by environmental exposure to agents which interfere with detection or repair and here it critical to best match environmental exposure to controlled laboratory conditions. Evidence exists that either neonatal or adult exposure to the weak estrogen bisphenol A (BPA) can induce aneuploidy in oocytes, suggesting environmental pollutants could underlie some age-related aneuploidy. Non-disjunction in mouse is an uncommon event in most strains, yet very high rates of aneuploidy can result when mice are exposed to BPA, a common constituent of polycarbonate plastics and resins used to line food cans and make dental sealants. Aneuploidy happens when oocytes are exposed during their maturation in vitro or when oocytes are exposed to excess BPA in the animals diet (Hunt et al., 2003; Can et al., 2005). Also exposure of fetal oocytes to BPA can affect the placement and number of recombination events on chromosomes, this probably generates susceptible patterns of exchange, and so leads to increased non-disjunction in treated animals (Susiarjo et al., 2007). It is suggested that BPA may interfere with the function of estrogen receptor beta (ERβ) at this time, given the same recombination phenotype is observed in ERβ knockout mice (Susiarjo et al., 2007). However, this is unexpected as it suggests a very early stimulatory effect of a fetal estrogen directly on oocyte recombination.
Exposure of maturing mouse oocytes to high levels of the gonadotropin FSH can also induce aneuploidy in vitro (Roberts et al., 2005). Such an observation is interesting from the perspective of circulating gonadotropins being higher in older women with diminishing ovarian reserve, and the observations that increased FSH has been measured in women having DS children (van Montfrans et al., 2002). This latter effect appears to be due to a decreased ovarian reserve. The influence of FSH on aneuploidy therefore requires much further investigation.
Completion of meiosis II at fertilization
The preovulatory LH surge induces oocyte maturation and subsequent re-arrest at metaphase of the second meiotic division just prior to ovulation. Arrest at MetII and subsequent fertilization at this stage of meiosis is universal in mammals, even in Canidae oocytes which are exceptional in being ovulated at the GV stage (Reynaud et al., 2006). Re-arrest is protracted and oocytes of most animals seem to show a very good block to further progression through MetII (Jones, 1998).
Cytostatic factor
Re-arrest is achieved by the development of an activity during oocyte maturation first described as ‘cytostatic factor’ (CSF) (Masui and Markert, 1971), which at molecular level is now thought likely to be expression in maturing oocytes of the APC inhibitor Emi2/Erp1(Liu and Maller, 2005; Rauh et al., 2005; Schmidt et al., 2005; Tung et al., 2005; Madgwick et al., 2006; Shoji et al., 2006). The degradation of this oocyte-specific protein at fertilization is triggered by its phosphorylation through the concerted activity of polo kinase and calmodulin-dependent kinase II (CamKII) (Madgwick et al., 2006; Schmidt et al., 2006). Although polo is already active in unfertilized oocytes, CamKII is not. Thus Emi2 is only fully phosphorylated and so degraded when CamKII is switched on at fertilization (Hansen et al., 2006); which in mammals is through an oscillatory Ca2+ signal induced by a sperm-specific member of the phospholipase C (PLC) family called PLC zeta (Saunders et al., 2002; Kouchi et al., 2004; Swann et al., 2006). Fully phosphorylated Emi2 is then degraded by the E3 ligase complex Skp1-Cullin/F-box protein. The mos/MAPkinase pathway has long been implicated also in MetII arrest (O’Keefe et al., 1989; Sagata et al., 1989). Recently, in frog it was demonstrated that the mos pathway is responsible for stabilizing Emi2 through p90 ribosomal S6 kinase (p90rsk) (Inoue et al., 2007; Nishiyama et al., 2007) so neatly establishing a link between the long-time CSF candidate mos and the much newer contender Emi2. Interestingly, p90rsk plays no part in arresting mouse oocytes at MetII, so it will be important to establish the link between the mos and Emi2 in mammals (Dumont et al., 2005), given mos clearly plays a role in arresting mammalian oocytes at MetII (Colledge et al., 1994; Hashimoto et al., 1994; Verlhac et al., 1996).
Degradation of Emi2 is responsible for the 6–7-fold increase in APC activity observed at fertilization (Nixon et al., 2002) that leads to rapid cyclin B1 proteolysis. Therefore a drop in CDK1 activity is an early event of fertilization. Also likely degraded is securin which is probably re-synthesized during MetII arrest, thus contributing to separase inhibition. However, it is also possible that the high CDK1 activity associated with MetII arrest inhibits separase, as has been observed in other cells (Gorr et al., 2005; Holland and Taylor, 2006). Interestingly, the binding of CDK1 to separase leads to a mutual inhibition of activities, with binding being thought to play an essential role in inhibiting CDK1 during MI (Gorr et al., 2006). Overexpression of non-degradable cyclin B1 in fertilizing oocytes blocks not only the decline in CDK1 activity, but also sister chromatid separation, suggesting that CDK1 can function as a separase inhibitor during MetII arrest (Madgwick et al., 2004); although whether this applies at physiological levels of CDK1 remains to be established.
The ability of ovulated oocytes to maintain a fully functional spindle with bi-orientated sisters is probably finite and would account for the drop in oocyte quality associated with post-ovulatory aging. Interestingly, increasing female age exacerbates the decline in oocyte quality with post-ovulatory aging and is probably accounted for by a decline in their ability to maintain high CDK1 activity (Tatone et al., 2006). Metabolic, biochemical and structural parameters also decline in oocytes undergoing post-ovulatory ageing (Takahashi et al., 2000; Igarashi et al., 2005; Huang et al., 2007) and the fertilization-associated calcium signal seems to switch on apoptotic pathways as oocytes age (Gordo et al., 2002). Finally, there appears important long-term detriment to the animal derived from a post-ovulatory aged oocyte, since both its reproductive fitness as an adult and its longevity are adversely affected (Tarin et al., 2002).
Aneuploidy in oocytes: is it a human condition?
Much of the present review has focussed on the mouse oocyte. Yet, many will argue that the human oocyte is uniquely prone to segregation errors in meiosis and therefore any other model system, including mouse, is inappropriate for the study of aneuploidy and in particular age-related aneuploidy. Such judgement may be erroneous. Even the evolutionary distant but important model organism Drosophila melanogaster can display increased incidence of age-related aneuploidy on a background where sister chromatid cohesion is perturbed (Jeffreys et al., 2003).
It is true that many strains of mice have low rates of aneuploidy and the above age-related aneuploidy in Drosophila requires disruption in the expression of the ORD gene product involved in sister chromatid cohesion. However, high, human-like levels of aneuploidy can exist without perturbation in some mice. For example, crosses of C57BL/6 with SPRET mice, generate F1 female mice with an aneuploidy rate of ∼10% at age 4 weeks, corresponding to a young adult, which then doubles to 20% in mice aged 8–11 months that are at the end of their reproductive life (Koehler et al., 2002). This increase in aneuploidy was associated with a decrease in recombination frequency between homologs and is likely due to sequence divergence in the homologs of these two strains given the same phenomenon can be observed in close strains of yeasts (Hunter et al., 1996). Also some strains of mice such as CBA display higher rates of aneuploidy than other strains (Eichenlaub-Ritter et al., 1988). As an aside it is important to note that achiasmate homologs are a feature of meiosis in a number of organisms, especially in Drosophila (Wolf, 1994; Thomas et al., 2005). One mechanism to account for proper segregation of achiasmate homologs in Drosophila is heterochromatin pairing (Karpen et al., 1996), such that non-disjunction is substantially increased when heterochromatin pairing is reduced by deleting heterochromatin content of an introduced mini-chromosome. It would be interesting to determine if pairing of homologs in mammalian oocytes, independent of chiasmata, play any part in MI as it can in other organisms (Gerton and Hawley, 2005).
Concluding remarks
This review has had two main purposes: first, to give a broad review of the cell biology responsible for the meiotic cell cycle transitions which define the remarkable life of an oocyte; second, to put our knowledge of cell biology into a clinical context by using it to discuss our current understanding of the unique susceptibility of the oocyte to aneuploidy. It is hoped that despite the fact that the etiology of aneuploidy is unlikely to be found in one particular meiotic defect or even pathway, our understanding of its causes will likely come from basic cell biology done on model organisms which are more tractable than human, and such knowledge will eventually feed into a clinical setting by collaborations of basic and applied researchers who together may find newer and better methods for prevention, screening and possible treatment. Major themes basic cell biologist are likely to make substantial progress in the next decade are (i) how germ cells commit to entry into meiosis; (ii) how homologs are held together for the maintenance of cohesion and how they may deteriorate with age; (iii) how the oocyte remains viable during a protracted period of GV arrest; (iv) how the oocyte controls the segregation of homologs during the first meiotic division and (v) how the oocyte maintains MetII arrested and how this is detrimentally affected by post-ovulatory ageing. Pursuit of answers to these questions will likely lead to a better understanding of aneuploidy in oocytes.
Funding
The author would like to acknowledge continued funding from the Wellcome Trust.