-
PDF
- Split View
-
Views
-
Cite
Cite
Charles S. Berenson, Mary Alice Garlipp, Lori J. Grove, Jane Maloney, Sanjay Sethi, Impaired Phagocytosis of Nontypeable Haemophilus influenzae by Human Alveolar Macrophages in Chronic Obstructive Pulmonary Disease, The Journal of Infectious Diseases, Volume 194, Issue 10, 15 November 2006, Pages 1375–1384, https://doi.org/10.1086/508428
Close - Share Icon Share
Abstract
BackgroundInteractions of nontypeable Haemophilus influenzae (NTHI) with human alveolar macrophages are implicated in the persistence of NTHI in chronic obstructive pulmonary disease (COPD). However, the immunologic mechanisms that mediate NTHI-induced macrophage responses are poorly understood. We hypothesized that immunologic responses of alveolar macrophages to NTHI are impaired in COPD
MethodsBlood and alveolar macrophages—obtained from ex-smokers with COPD (n=14), ex-smokers without COPD (n=15), and nonsmokers (n=9)—were incubated with 3 distinct NTHI strains obtained from patients with COPD. Phagocytosis of 3H-NTHI, expressed as a percentage of the mean total radioactivity, and of intracellular viability, assessed as a percentage of viable cell-associated NTHI, were measured
ResultsAlveolar macrophages from donors with COPD, compared with those from donors without COPD, had impaired phagocytosis (median [interquartile range]) for each NTHI strain: 14P13H5, 0.26 (0.08–0.61) versus 1.36 (0.69–1.95); 6P5H1, 0.92 (0.32–1.82) versus 1.90 (1.32–2.68); and 14P14H1, 0.79 (0.23–1.32) versus 2.13 (1.13–2.40) (P⩽.01 for each). However, phagocytosis of all NTHI strains by blood macrophages from donors with COPD was indistinguishable from that of blood macrophages from donors without COPD and from nonsmokers. The intracellular killing of NTHI was not impaired in alveolar macrophages from donors with COPD
ConclusionsThese results support a paradigm of impaired phagocytosis by alveolar macrophages, but not blood macrophages, in COPD and provide an immunologic basis for persistence of NTHI in the airways of adults with COPD
Nontypeable Haemophilus influenzae (NTHI) are the most common pathogenic bacteria isolated from the airways of patients with chronic obstructive pulmonary disease (COPD) [1]. The relationship between NTHI and the progression of COPD is underscored by an increased risk of exacerbation associated with the acquisition of new respiratory strains of NTHI [2]. Bacterial colonization is associated with advanced airway inflammation, increased frequency of exacerbations, and an accelerated decrease in lung function, which strongly suggests that chronic airway inflammation induced by bacterial colonization of the lower airways in COPD contributes to progressive lung disease [3–6]. Once initiated, chronic inflammation and failure to clear bacteria from the lower airways may promote the progression of COPD [7]
Macrophages are critical to host defense against NTHI [8, 9]. The intracellular survival of NTHI after phagocytosis has been considered to play a role in bacterial persistence and pathologic changes. Examination of hypertrophied adenoid tissue removed at adenoidectomy from 10 children revealed intracellular NTHI in all 10 adenoids. NTHI were viable and actively dividing in mononuclear cells, each of which contained up to 200 intracellular NTHI [10]. Examination of explanted lungs from patients undergoing lung transplantation detected NTHI in 8 of 16 participants in whom the underlying disease was COPD [11]. In fact, NTHI was present in tissue sections of 47% of explants from COPD patients compared with only 29% of those with other pulmonary diseases (P<.001). Craig et al. [12] found in vitro survival of NTHI strains for 72 h after phagocytosis in J774 murine macrophages. Of 33 clinical otitis media isolates of NTHI, 27 remained viable after phagocytosis [12]. Macrophages are also effective in vivo phagocytes of NTHI in animal models [13]. Although these results support the idea that macrophages play a role in the clearance of NTHI infections, most studies have focused on nonhuman macrophages and diseases other than COPD, leaving unanswered questions about the interaction between macrophages and COPD bacterial isolates. Furthermore, the inclusion of active smokers in some studies of COPD has added a potentially confounding variable to studies of macrophage function. One study of phagocytosis of NTHI by alveolar macrophages from smokers demonstrated increased bactericidal activity, compared with alveolar macrophages from nonsmokers [14]
We recently found impaired proinflammatory responses to purified immunoactive antigens of NTHI in alveolar macrophages from former smokers with COPD, independent of active smoking. These findings suggest an alternate paradigm of generalized alveolar macrophage dysfunction, including phagocytosis, as an immunologic basis for the persistence of NTHI in COPD [15]. To determine whether dysfunctional phagocytosis or impaired intracellular killing in COPD alveolar macrophages plays a role in permitting NTHI to evade host responses, we obtained alveolar and blood macrophages from ex-smokers with and without COPD and performed experiments to test these hypotheses
Subjects, Materials, and Methods
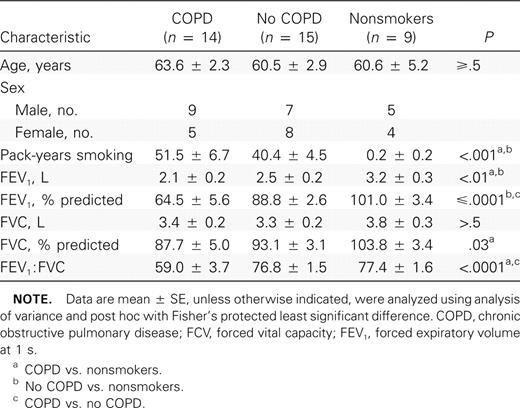
Recruitment of subjectsAll procedures received approval from the institutional review board of the VA Western New York Healthcare System. Participants were at least 30 years old. After they provided informed consent, volunteers were screened for inclusion in 1 of 3 groups: (1) ex-smokers with COPD, (2) ex-smokers without COPD, and (3) nonsmokers. All subjects underwent clinical assessment, routine spirometry, and chest x-ray and had expired breath carbon monoxide levels of ⩽0.02 ppm (Vitalograph Breath CO Monitor; Lenexa)
For the COPD group, exclusion criteria included a forced expiratory volume at 1 s (FEV1) of <35%, hypercapnia, and comorbid diseases that would render bronchoscopy unsafe. Inclusion criteria were (1) chronic bronchitis as determined by history and/or emphysema as determined by chest x-ray or computed tomography; (2) ⩾20 pack-years of cigarette smoking but smoking cessation for at least 1 year preceding enrollment; (3) the absence of other lung disease, including asthma and bronchiectasis; (4) chest x-ray findings that were normal or compatible with COPD but no other disease detected; (5) FEV1:forced vital capacity ratio and FEV1 both below the lower 95% confidence limit of normal on spirometry; (6) no atopy in the medical history; (7) <15% bronchodilator response with inhaled albuterol on spirometry; and (8) no antibiotic or steroid use for 4 weeks preceding enrollment
Inclusion criteria for the no-COPD group were the same as those for the COPD group, except for the absence of lung disease by clinical evaluation, normal chest x-ray, and normal spirometric results. Healthy nonsmokers met all inclusion criteria for the no-COPD group, except that all had <5 cumulative pack-years of smoking
ReagentsRPMI1640 was purchased from BioWhittaker. Human AB-positive serum was purchased from Nabi
Purification of human blood monocyte–derived macrophagesWhole blood (25–50 cm3) was obtained from each subject and placed in heparinized containers. Peripheral blood mononuclear cells were purified by density centrifugation at 400 g and seeded onto 48-well Linbro tissue-culture plates (Hampton Research) at 1×106 cells/well [16]. After incubation for 7–10 days, nonadherent cells were removed
Purification of human alveolar macrophagesAll participants underwent bronchoalveolar lavage (BAL) so that we could obtain alveolar macrophages, as detailed elsewhere [15]. The right middle lobe was lavaged 3 times with sterile saline (50 mL). Follow-up was done 24 h after bronchoscopy
Alveolar macrophages were purified from individual BAL samples and seeded onto 48-well Linbro tissue-culture plates (1×105 cells/mL) in RPMI 1640/10% AB-positive serum [15]. After incubation (in 5% CO2 and 95% humidity at 37°C) for 24 h, nonadherent cells were removed. The remaining alveolar macrophages were consistently 98%–100% esterase positive [17]
Bacterial strainsThree distinct clinical strains of NTHI were obtained from sputum samples from subjects with COPD who were monitored prospectively in our COPD study clinic and provided monthly sputum samples. Strain 1 (14P13H5), present in only 1 sputum sample at a single visit, was deemed to be a short-term colonizer. Strain 2 (6P5H1) was newly acquired from a subject with clinical symptoms of COPD exacerbation. Strain 3 (14P14H1) was repeatedly isolated from sputum samples over the course of 1 year. Conditions for incubation of each NTHI strain with human macrophages were first established by incubation in macrophage-free wells, under conditions identical to those of subsequent experiments, to confirm that reagents, including antibody-depleted serum, would not be lethal. Antibody-depleted human serum was purified by Protein G affinity chromatography (Amersham), as described elsewhere [18]
Phagocytosis of NTHIThe assay for phagocytosis was adapted from published methods to be performed with adherent cells, rather than in suspension [8, 19]. Each NTHI strain was inoculated in brain-heart infusion supplemented with bovine hemin and NAD (10 μg/μL). 3H-leucine (3H; specific activity, 117–173 Ci/mmol; Perkin-Elmer) was added to bacteria (final concentration, 260 μCi/mL). Bacteria were grown to the midlogarithmic phase, transferred to 10 mL of Gey’s balanced salt solution (BSS), supplemented with 10% bovine serum albumin (BSA; Sigma Chemical), and centrifuged (5000 g for 15 min at 4°C). Bacteria were washed 3 times and resuspended in 1 mL of Gey’s BSS and 10% BSA. The incorporation of 3H into bacterial proteins was confirmed by SDS-PAGE autoradiography. An aliquot of 3H-NTHI confirmed the consistent incorporation of 0.5%–1% of 3H. 3H-NTHI were incubated with macrophages (220:1) in 6%–8% antibody-depleted serum for 1 h at 37°C in 5% CO2. After incubation, supernatants were removed and saved for 3H counting. Residual extracellular bacteria were killed by the addition of 100 μg/mL gentamicin (Sigma Chemical) to each well (30 min). Intracellular NTHI were recovered by lysis of cells in 0.5 mL of pyrogen-free water with sonication. Cell lysates were solubilized in scintillation fluid (Perkin-Elmer) and counted for 3H. The mean cell-associated radioactivity is expressed as a percentage of the mean total radioactivity of each well, calculated as follows: phagocytosis index = (intracellular cpm/intracellular + extracellular cpm) × 100. To confirm the elimination of extracellular NTHI with gentamicin and the viability of NTHI strains, each experiment included control wells of macrophage-free NTHI treated identically to those in experimental wells. Supernatants and residual bacteria were recovered and counted for 3H along with experimental samples
Intracellular survival of NTHIUsing the same procedure described for phagocytosis, macrophages were incubated with the same 3 strains of NTHI but not labeled with 3H. After incubation with macrophages, supernatants were removed, and extracellular bacteria were killed by incubation with gentamicin (100 μg/mL). For these studies, cell lysates and supernatants of each well were plated on chocolate agar, incubated overnight at 35°C in dilutions of 1:1–1:10, and evaluated by colony counts of cell lysates rather than by scintillation counting. Intracellular survival is expressed as a percentage of viable cell-associated NTHI of 3 separate wells, calculated as follows: survival index = (intracellular colonies/intracellular + extracellular colonies) × 100. To confirm the elimination of extracellular NTHI with gentamicin and the viability of NTHI, each experiment included control wells of macrophage-free NTHI, treated identically to those in experimental wells. Supernatants and residual bacteria were recovered and plated for colony counts
Statistical analysisDemographic data were normally distributed and were analyzed using analysis of variance and with Fisher’s test for multiple comparisons. Demographic data are expressed as means ± SEs (table 1). Intra-assay variability for phagocytosis and intracellular survival was consistently <10% for each NTHI strain. Median values of triplicate or quadruplicate samples were used for subsequent group analyses. Phagocytosis and intracellular survival data among groups were not normally distributed and are reported as median (interquartile range), as determined by Kruskal-Wallis and Mann-Whitney U tests. For all comparisons, P<.05 was considered to be significant
Results
Recruitment of participantsOf 49 volunteers recruited, 10 were not included: 2 were excluded because of the presence of comorbid disease, 4 later decided not to participate, and 4 did not meet entry criteria. Of 39 qualified participants, all underwent successful bronchoscopies. Data from 1 subject were not evaluable because of inadequate cell numbers
The 38 subjects whose data were evaluable for the study included 21 men and 17 women. There were 14 participants in the COPD group, 15 in the no-COPD group, and 9 in the nonsmoker group. Demographic and pulmonary function data are presented in table 1. As expected, subjects with COPD had significantly worse spirometric parameters (FEV1 and percentage predicted FEV1) than subjects in the no-COPD and nonsmoker groups. However, differences in cumulative tobacco exposure were not significant between subjects in the COPD and no-COPD groups. There were no significant intergroup differences in age (table 1). Seven members of the COPD group were receiving bronchoactive medications, including inhaled corticosteroids (5), β-agonists (7), and anticholinergic medications (6)
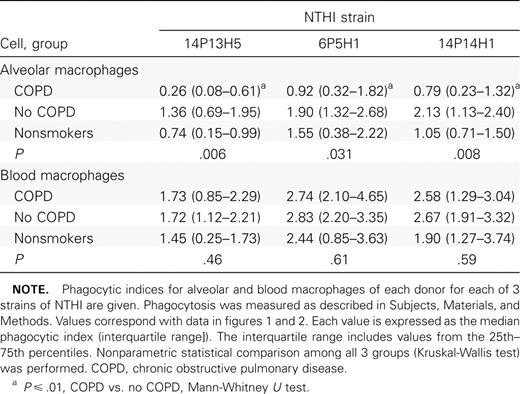
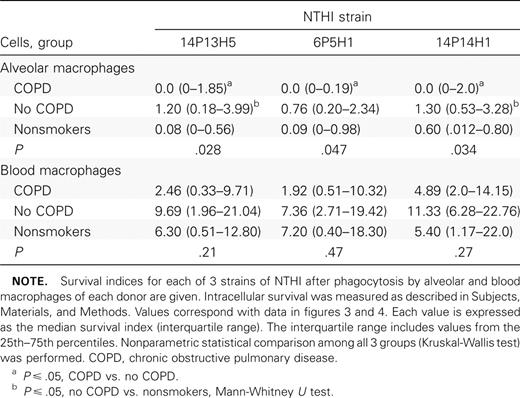
Phagocytosis of NTHI by alveolar macrophagesTo investigate the relative phagocytic capabilities of alveolar macrophages from each group, alveolar macrophages were incubated with each of 3 clinical radiolabeled strains of NTHI and evaluated for the incorporation of radiolabel, measured as counts per minute. To accommodate for variation between wells and between experiments, intracellular counts per minute were normalized against counts per minute of cell supernatants, and a phagocytic index was calculated as described in Subjects, Materials, and Methods. For each donor, quadruplicate values for each strain were averaged to arrive at a final phagocytic index. Data (table 2) are expressed as median (interquartile range)
Phagocytosis of nontypeable Haemophilus influenzae (NTHI) by human alveolar and blood macrophages
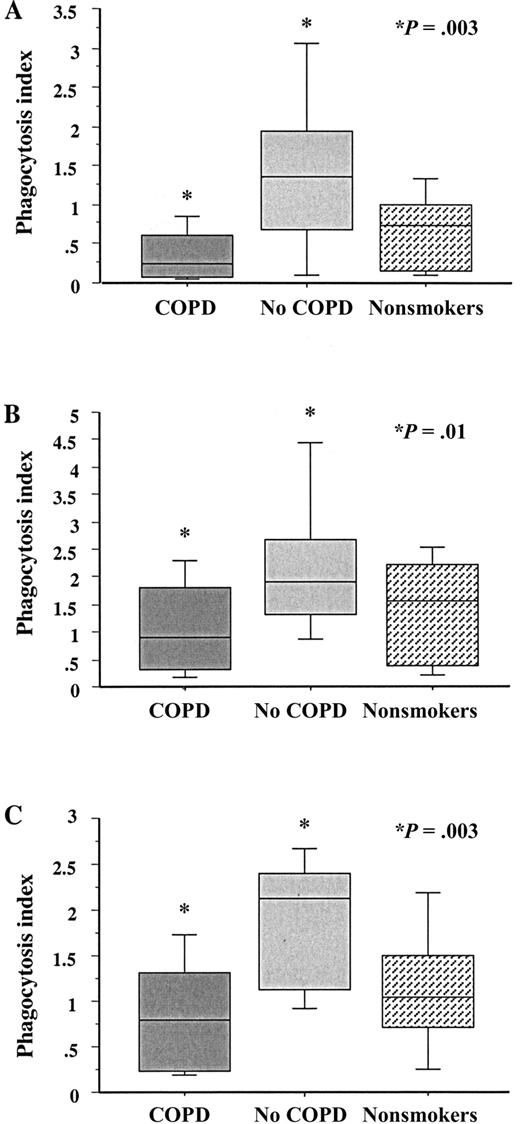
The greatest overall difference between groups was between alveolar macrophages from ex-smokers with COPD and those without COPD for all 3 NTHI strains (figure 1; table 2). For example, the phagocytic index of alveolar macrophages from subjects with COPD, compared with those from subjects without COPD, was 0.26 (0.08–0.61) versus 1.36 (0.69–1.95) for NTHI strain 14P13H5 (P=.003). The same comparison was true for the other NTHI strains. In each instance, the phagocytic index was statistically lower for alveolar macrophages from subjects with COPD (P⩽.01 for each). There was no statistical difference between phagocytic indices for alveolar macrophages from nonsmokers and those from the other 2 groups. There was no statistical correlation between the use of any bronchoactive medications and impaired alveolar macrophage phagocytosis
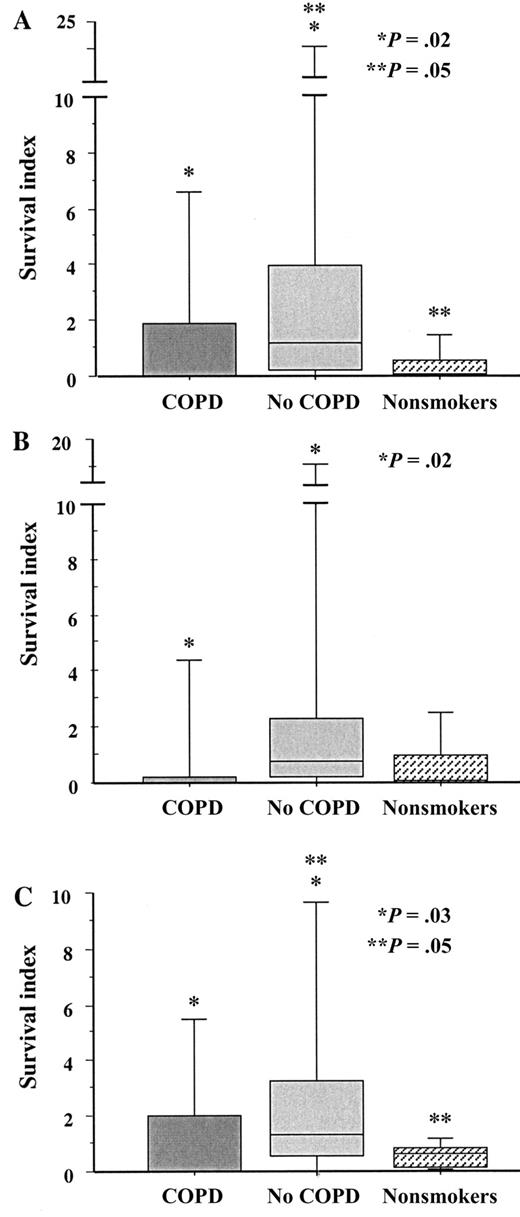
Phagocytosis of nontypeable Haemophilus influenzae (NTHI) by human alveolar macrophages. Alveolar macrophages were obtained from former smokers with chronic obstructive pulmonary disease (COPD; dark gray shading), former smokers without COPD (light gray shading) and nonsmokers (stippled shading). Cells were incubated with 3 distinct clinical strains of NTHI. A Strain 14P13H5; B strain 6P5H1; C strain 14P14H1. Phagocytosis was measured as described in Subjects, Materials, and Methods. Results are shown as box plots for each group. Each box encompasses the 25th–75th interquartile range, and the horizontal line in each box represents the median value. Each vertical bar encompasses the 10th–90th percentile range. Data correspond to values in table 2. Statistical comparisons for all 3 groups were performed using the Kruskal-Wallis test. Intergroup comparisons, for which P values are shown, were performed using the Mann-Whitney U test
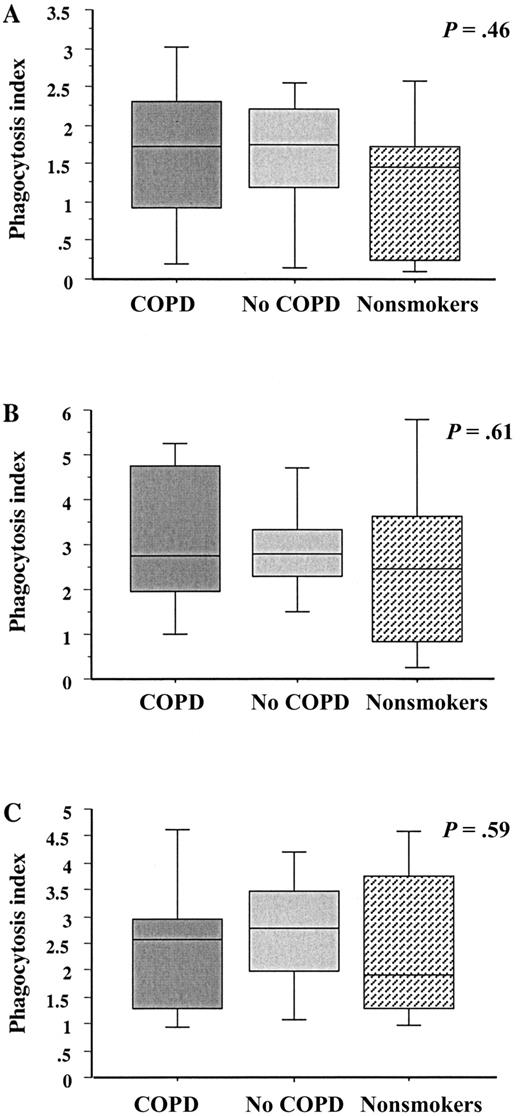
Phagocytosis of NTHI by blood macrophagesTo determine whether the relative impairment of phagocytosis in COPD was limited to alveolar macrophages, blood monocyte–derived macrophages from subjects in each group were incubated with each radiolabeled NTHI strain, as described above. Analysis of phagocytosis was done as was described for alveolar macrophages. For each strain, there were no statistically significant differences in phagocytosis among macrophages from any group (figure 2; table 2). Phagocytosis of NTHI by blood macrophages from subjects with COPD was no different from that by blood macrophages from subjects in the no-COPD and nonsmoker groups (P>.4 for each)
Phagocytosis of nontypeable Haemophilus influenzae (NTHI) by human blood macrophages. Blood monocyte–derived macrophages were obtained from former smokers with chronic obstructive pulmonary disease (COPD; dark gray shading), former smokers without COPD (light gray shading) and nonsmokers (stippled shading). Cells were incubated with 3 distinct clinical strains of NTHI. A Strain 14P13H5; B strain 6P5H1; C strain 14P14H1. Phagocytosis was measured for each strain. Data are represented by box plots for each group, as detailed in figure 1. Data correspond to values in table 2. Statistical comparisons for all 3 groups, for which P values are shown, were performed using the Kruskal-Wallis test
Intracellular survival of NTHI in alveolar macrophages To investigate the ability of NTHI to survive intracellularly in alveolar macrophages from subjects with COPD, alveolar macrophages were incubated with each NTHI strain and evaluated for postphagocytosis bacterial viability. As in earlier experiments, to accommodate for variation between wells and between experiments, colony counts of intracellular bacteria were normalized against counts of viable colonies recovered from cell supernatants. For each donor, triplicate values for each strain were averaged to arrive at a final survival index, calculated as described in Subjects, Materials, and Methods. Data are expressed as median (interquartile range)
For NTHI strain 6P5H1, there were statistically fewer intracellular survivors in alveolar macrophages from subjects in the COPD group than in the those from subjects in the no-COPD group (P=.02) (figure 3; table 3). The survival indices of NTHI strains 14P13H5 (P=.02) and 14P14H1 (P=.03) were also significantly lower in alveolar macrophages from the COPD group than in those from the no-COPD group and were marginally different in alveolar macrophages from the nonsmoker group, compared with those from the no-COPD group (P=.05 for each) (figure 3). The diminished number of viable NTHI in the COPD alveolar macrophages may, in part, be a function of impaired phagocytosis
Intracellular survival of nontypeable Haemophilus influenzae (NTHI) in human alveolar macrophages. Alveolar macrophages were obtained from former smokers with chronic obstructive pulmonary disease (COPD), former smokers without COPD, and nonsmokers. Shading denoting each individual group is as described in figure 1. Cells were incubated with the 3 clinical strains of NTHI detailed in figure 1. A Strain 14P13H5; B strain 6P5H1; C strain 14P14H1. Intracellular survival was measured as described in Subjects, Materials, and Methods. Data are represented by box plots for each group, as detailed in figure 1. Data correspond to values in table 3. Statistical comparisons for all 3 groups were performed using the Kruskal-Wallis test. Intergroup comparisons, for which P values are shown, were performed using the Mann-Whitney U test
Intracellular survival of nontypeable Haemophilus influenzae (NTHI) in human alveolar and blood macrophages
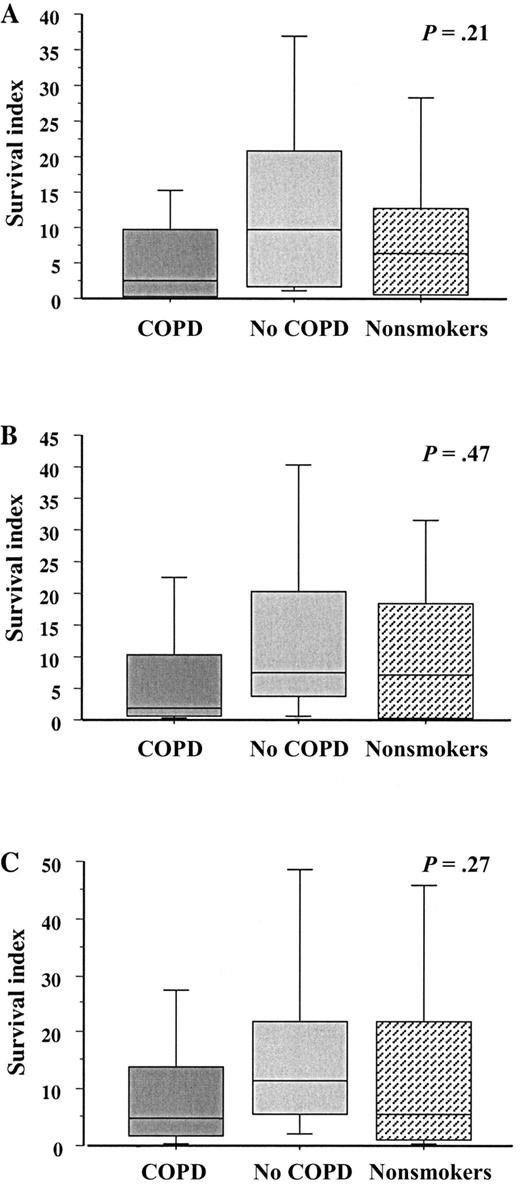
Intracellular survival of NTHI in blood macrophagesTo investigate the comparative intracellular survival of NTHI in blood macrophages from subjects with COPD, blood macrophages were incubated with each NTHI strain as above and evaluated for postphagocytosis bacterial viability. Analysis of intracellular NTHI survival was done as was described for alveolar macrophages. For each NTHI strain, there were no statistically significant differences in intracellular survival among macrophages from any group (P>.2 for each) (figure 4; table 3)
Intracellular survival of nontypeable Haemophilus influenzae (NTHI) in human blood macrophages. Blood monocyte–derived macrophages were obtained from former smokers with chronic obstructive pulmonary disease (COPD), former smokers without COPD, and nonsmokers. Shading denoting each individual group is as described in figure 1. Cells were incubated with the 3 clinical strains of NTHI detailed in figure 1. A Strain 14P13H5; B strain 6P5H1; C strain 14P14H1. Intracellular survival of each strain was measured. Data are represented by box plots for each group, as detailed in figure 1. Data correspond to values in table 3. Statistical comparisons for all 3 groups, for which P values are shown, were performed using the Kruskal-Wallis test
Discussion
To our knowledge, the present study is the first to demonstrate a fundamental phagocytic defect for NTHI in alveolar macrophages from persons with COPD and to investigate comparative phagocytic responses of same-donor alveolar and blood macrophages. The presence of phagocytic dysfunction of alveolar macrophages, but not of blood macrophages, in subjects with COPD suggests a compartmentalized immunologic defect. Although it is difficult to be certain whether the lower numbers of intracellular NTHI survivors in alveolar macrophages from subjects with COPD is a function of diminished phagocytosis or more-potent intracellular killing, our findings do not support defective intracellular killing as a feature of COPD alveolar macrophages. Although the present study—coupled with our recent findings of impaired inflammatory cytokine responsiveness to NTHI antigens of alveolar macrophages from subjects with COPD—supports a model of broad immunologic dysfunction of alveolar macrophages in COPD, the contribution of alveolar macrophage dysfunction to COPD remains speculative [15]
Our assays for phagocytosis and intracellular survival were intentionally modified from methods published elsewhere, to prevent the methods from artifactually contributing to the impairment of macrophage function [8, 19]. Each assay was performed in the same culture wells in which macrophages were adherent, rather than removing macrophages in suspension, to minimize the disruption of macrophages. The incubation of NTHI-infected macrophages with gentamicin, to eliminate extracellular NTHI, permitted the quantitation of intracellular NTHI. Essential control wells in each experiment included NTHI without macrophages, treated with and without gentamicin, to verify the viability of NTHI and the elimination of extracellular NTHI with gentamicin. One limitation of this method may be the relatively small numbers of intracellular NTHI recovered. However, this was calculated in relation to extracellular NTHI recovered, which permitted accurate comparisons among patients and among groups
Macrophage interactions are critical not only to the progression of NTHI-related disease, but of COPD in particular [20, 21]. The immunologic activities of macrophages can contribute to many features of COPD and, thus, may play roles in the pathogenesis of COPD, as well as in its associated symptoms [22, 23]. The intrinsic dysfunction of alveolar macrophages in COPD is exemplified by the diminished expression of histone deacetylase in alveolar macrophages from patients with COPD, compared with others [24]. Earlier investigations with nonbacterial targets indicated that alveolar macrophages from persons with COPD may have diminished phagocytic abilities [25]. We specifically chose human macrophages as effector cells because NTHI is a strictly human pathogen [26]. We further chose alveolar macrophages because NTHI is predominantly a respiratory pathogen. Although important immunologic mechanisms have arisen from investigations of interactions between NTHI and blood macrophages, studies with other Haemophilus species have indicated that the phagocytic capabilities of mammalian alveolar and blood macrophages may be discordant [27, 28]
NTHI is a heterogeneous grouping of bacteria that has an integral relationship with COPD. NTHI not only is more prevalent in COPD but also has been implicated in the pathogenesis of COPD. The acquisition of new respiratory strains of NTHI is associated with an increased risk of exacerbations [2]. Proliferative lymphocyte responses, evoked in vitro with the NTHI outer-membrane protein (OMP) P6, correlate with relative protection against COPD exacerbations in humans [29]. In fact, in vivo strain-specific antibody responses to NTHI are also associated with COPD exacerbations [30]. Because NTHI is heterogeneous, we selected 3 distinct NTHI strains, obtained from patients with COPD under different clinical circumstances, to determine whether defective phagocytosis is specific for individual strains [31]. Although the characterization of the individual strains was limited, we found no strain-related difference in phagocytic capabilities of alveolar macrophages from persons with COPD. Because our findings are limited to NTHI, it is not possible to conclude whether impaired alveolar macrophage phagocytosis in cells from persons with COPD is specific for NTHI. However, related studies have demonstrated diminished phagocytic abilities of alveolar macrophages from persons with COPD, as well as of apoptotic airway cells, during Candida albicans infection [25, 32]
The mechanisms underlying alveolar macrophage dysfunction in COPD are a key focus of our ongoing studies. The phagocytosis of microbial pathogens is remarkably complex and diverse and is governed by many cellular interactions [33]. Numerous signaling pathways are activated with microbial contact to initiate a phagocytic response [34, 35]. Although opsonins appear to be critical for the phagocytosis of type b H. influenzae it is not clear whether this also applies to NTHI [8, 19]. Persistent exposure to bacteria can regulate the expression of immunoregulatory surface molecules, which results in relative tolerance by alveolar macrophages [36]. Although this expression has been described in Toll-like receptors of murine alveolar macrophages and THP-1 cells, its function in human alveolar macrophages is speculative [37, 38]. It is conceivable that impaired phagocytosis of NTHI by alveolar macrophages from persons with COPD is mediated by persistent exposure and compensatory down-regulation. Our related findings of impaired cytokine responsiveness to OMP P6 and lipo-oligosaccharide of NTHI further suggest that intrinsic alveolar macrophage impairment in COPD is not limited to a single intracellular signaling pathway [15]
Several studies that provided key insights into underlying immunologic mechanisms in COPD included active smokers. For example, the determination of interleukin (IL)–8 levels in BAL samples as a distinguishing marker of COPD included active smokers, which implicated smoking as a potential independent contributor to the immune induction of IL-8 in COPD [39]. Alveolar macrophages in COPD are also less responsive to the suppressive effects of corticosteroids, although this too may be influenced by active smoking [40]. In fact, a study of the diminished expression of histone deacetylase in alveolar macrophages from persons with COPD also included active smokers [24]. Phagocytosis of NTHI by alveolar macrophages from smokers may be accompanied by distinct functional differences, compared with alveolar macrophages from nonsmokers [14]. Therefore, in the present study, as well as in our previous study, the omission of active smokers permitted us to conclude that alveolar macrophage dysfunction in COPD is independent of active smoking [15]
Results of studies of phagocytic dysregulation that rely on murine and human nonalveolar macrophages may not readily apply to COPD. Studies with murine J774 macrophages, as well as with human adenoidal tissue, both demonstrated pronounced persistence and viability of clinical isolates of NTHI after phagocytosis [10, 12]. Yet our results when we used human alveolar macrophages did not detect any defective intracellular killing of any NTHI strains by alveolar macrophages from persons with COPD to account for the persistence of NTHI in COPD
There are inherent limitations to our study as well. Although biologically appealing, the use of human cells involves greater variability and overlap between donors than might be found when cell lines are used. The relatively small number of participants in each group stems from recruitment of volunteers for research that entailed an invasive procedure and excluded active smokers. However, the statistically significant differences between groups in biologically induced phagocytic function in alveolar macrophages from ex-smokers with COPD, compared with ex-smokers without COPD, and the absence of differences between groups in phagocytic responses by blood macrophages, should allay these concerns. The determination of defective phagocytosis of alveolar macrophages for each of 3 clinically distinct strains of NTHI raises questions about the phagocytic capabilities of alveolar macrophages from persons with COPD for other respiratory pathogens and inert targets, to which we are directing our ongoing studies. Although distinct phagocytic differences are evident between alveolar macrophages from subjects with and without COPD, it is intriguing that alveolar macrophages from nonsmokers were not statistically different from those from either group (figure 1). We suspect that this is in part because subjects with and without COPD were a select groups of individuals, whereas nonsmokers, who have not been exposed to long-term cigarette smoke, were unselected
The vicious-circle hypothesis has been proposed to explain the contribution of bacterial colonization of the lower airways to the pathogenesis of COPD [7]. Once initiated, bacterial colonization may perpetuate inflammation of the lower airways, which contributes to the progression of COPD [7]. Failure to evoke a phagocytic response by alveolar macrophages in COPD would permit the evasion of immune clearance by bacterial pathogens and provide a source for the perpetuation of inflammation. Alternatively, the phagocytic differences between alveolar macrophages from persons with COPD, compared with those without COPD, toward NTHI may be a key contributing factor to susceptibility to the effects of smoke exposure and the subsequent development of COPD. Collectively, these results demonstrate an impaired immunologic profile of alveolar macrophages, but not blood macrophages, in ex-smokers with COPD and provide an immunologic basis for the persistence of NTHI in COPD
Acknowledgments
We thank Dr. Timothy F. Murphy, for scientific advice; and Jane M. Smigiera, for technical assistance
References
Presented in part: 104th meeting of the American Society for Microbiology, New Orleans, 23–27 May 2004 (abstract B-420) and 105th meeting of the American Society for Microbiology, Atlanta, 5–9 June 2005 (abstract B-296)
Potential conflicts of interest: none reported
Financial support: National Institutes of Health (grant 1RO1HL6654901 to C.S.B. and S.S.); Department of Veterans Affairs