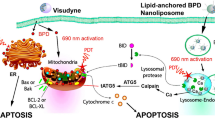
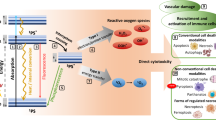
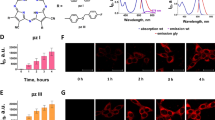
Abstract
Damage-associated molecular patterns (DAMPs) or cell death associated molecular patterns (CDAMPs) are a subset of endogenous intracellular molecules that are normally hidden within living cells but become either passively released by primary and secondary necrotic cells or actively exposed and secreted by the dying cells. Once released, DAMPs are sensed by the innate immune system and act as activators of antigen-presenting cells (APCs) to stimulate innate and adaptive immunity. Cancer cells dying in response to a subset of conventional anticancer modalities exhibit a particular composition of DAMPs at their cell surface, which has been recently shown to be vital for the stimulation of the host immune system and the control of residual disease. Photodynamic therapy (PDT) for cancer has long been shown to be capable of killing malignant cells and concomitantly stimulate the host immune system, properties that are likely linked to its ability of inducing exposure/release of certain DAMPs. PDT, by evoking oxidative stress at specific subcellular sites through the light activation of organelle-associated photosensitizers, may be unique in incorporating tumour cells destruction and antitumor immune response in one therapeutic paradigm. Here we review the current knowledge about mechanisms and signalling cascades leading to the exposure of DAMPs at the cell surface or promoting their release, the cell death mechanism associated to these processes and its immunological consequences.We also discuss how certain PDT paradigms may yield therapies that optimally stimulate the immune system and lead to the discovery of new DAMPs.
Similar content being viewed by others
References
M. E. Bianchi, DAMPs, PAMPs and alarmins: all we need to know about danger, J. Leukocyte Biol., 2006, 81, 1–5.
A. D. Garg, D. Nowis, J. Golab, P. Vandenabeele, D. V. Krysko and P. Agostinis, Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation, Biochim. Biophys.Acta, 2010, 1805, 53–71.
S. Y. Seong and P. Matzinger, Hydrophobicity: an ancient damageassociated molecular pattern that initiates innate immune responses, Nat. Rev. Immunol., 2004, 4, 469–478.
P. Matzinger, Tolerance, danger, and the extended family, Annu. Rev. Immunol., 1994, 12, 991–1045.
J. J. Maher, DAMPs ramp up drug toxicity, J. Clin. Invest., 2009, 119, 246–249.
C. S. Calfee and M. A. Matthay, Clinical immunology: Culprits with evolutionary ties, Nature, 2010, 464, 41–42.
S. S. Iyer, W. P. Pulskens, J. J. Sadler, L. M. Butter, G. J. Teske, T. K. Ulland, S. C. Eisenbarth, S. Florquin, R. A. Flavell, J. C. Leemans and F. S. Sutterwala, Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20388–20393.
A. D. Garg, D. Nowis, J. Golab and P. Agostinis, Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity, Apoptosis, 2010, 15, 1050–1071.
D. V. Krysko and P. Vandenabeele, Clearance of dead cells: mechanisms, immune responses and implication in the development of diseases, Apoptosis, 2010, DOI: 10.1007/s10495-10010-10524-10496.
M. Korbelik, PDT-associated host response and its role in the therapy outcome, Lasers Surg. Med., 2006, 38, 500–508.
M. Korbelik and J. Sun, Photodynamic therapy-generated vaccine for cancer therapy, Cancer Immunol. Immunother., 2005, 55, 900–909.
D. R. Green, T. Ferguson, L. Zitvogel and G. Kroemer, Immunogenic and tolerogenic cell death, Nat. Rev. Immunol., 2009, 9, 353–363.
L. Zitvogel, O. Kepp and G. Kroemer, Decoding cell death signals in inflammation and immunity, Cell, 2010, 140, 798–804.
L. Zitvogel, O. Kepp, L. Senovilla, L. Menger, N. Chaput and G. Kroemer, Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway, Clin. Cancer Res., 2010, 16, 3100–3104.
S. Demaria, E. Pikarsky, M. Karin, L. M. Coussens, Y. C. Chen, E. M. El-Omar, G. Trinchieri, S. M. Dubinett, J. T. Mao, E. Szabo, A. Krieg, G. J. Weiner, B. A. Fox, G. Coukos, E. Wang, R. T. Abraham, M. Carbone and M. T. Lotze, Cancer and inflammation: promise for biologic therapy, J. Immunother., 2010, 33, 335–351.
T. Panaretakis, O. Kepp, U. Brockmeier, A. Tesniere, A. C. Bjorklund, D. C. Chapman, M. Durchschlag, N. Joza, G. Pierron, P. van Endert, J. Yuan, L. Zitvogel, F. Madeo, D. B. Williams and G. Kroemer, Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death, EMBO J., 2009, 28, 578–590.
T. Verfaillie, A. D. Garg and P. Agostinis, Targeting ER stress induced apoptosis and inflammation in cancer, Cancer Lett., 2010, DOI: 10.1016/j.canlet.2010.07.016.
S. Hummasti and G. S. Hotamisligil, Endoplasmic reticulumstress and inflammation in obesity and diabetes, Circ. Res., 2010, 107, 579–591.
G. S. Hotamisligil, Endoplasmic reticulum stress and the inflammatory basis of metabolic disease, Cell, 2010, 140, 900–917.
A. Kaser and R. S. Blumberg, Endoplasmic reticulum stress and intestinal inflammation, Mucosal Immunol., 2009, 3, 11–16.
M. Obeid, A. Tesniere, F. Ghiringhelli, G. M. Fimia, L. Apetoh, J. L. Perfettini, M. Castedo, G. Mignot, T. Panaretakis, N. Casares, D. Metivier, N. Larochette, P. van Endert, F. Ciccosanti, M. Piacentini, L. Zitvogel and G. Kroemer, Calreticulin exposure dictates the immunogenicity of cancer cell death, Nat. Med., 2006, 13, 54–61.
R. Q. Peng, Y. B. Chen, Y. Ding, R. Zhang, X. Zhang, X. J. Yu, Z. W. Zhou, Y. X. Zeng and X. S. Zhang, Expression of calreticulin is associated with infiltration of T-cells in stage IIIB colon cancer, World J. Gastroenterol., 2010, 16, 2428–2434.
D. Jocham, A. Richter, L. Hoffmann, K. Iwig, D. Fahlenkamp, G. Zakrzewski, E. Schmitt, T. Dannenberg, W. Lehmacher, J. von Wietersheim and C. Doehn, Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial, Lancet, 2004, 363, 594–599.
M. May, S. Brookman-May, B. Hoschke, C. Gilfrich, F. Kendel, S. Baxmann, S. Wittke, S. T. Kiessig, K. Miller and M. Johannsen, Ten-year survival analysis for renal carcinoma patients treated with an autologous tumour lysate vaccine in an adjuvant setting, Cancer Immunol. Immunother., 2009, 59, 687–695.
E. A. Hirschowitz, T. Foody, R. Kryscio, L. Dickson, J. Sturgill and J. Yannelli, Autologous dendritic cell vaccines for non-small-cell lung cancer, J. Clin. Oncol., 2004, 22, 2808–2815.
L. M. Liau, R. M. Prins, S. M. Kiertscher, S. K. Odesa, T. J. Kremen, A. J. Giovannone, J.W. Lin, D. J. Chute, P. S. Mischel, T. F. Cloughesy and M. D. Roth, Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment, Clin. Cancer Res., 2005, 11, 5515–5525.
K. Palucka, H. Ueno, G. Zurawski, J. Fay and J. Banchereau, Building on dendritic cell subsets to improve cancer vaccines, Curr. Opin. Immunol., 2010, 22, 258–263.
C. L. Chiang, F. Benencia and G. Coukos, Whole tumor antigen vaccines, Semin. Immunol., 2010, 22, 132–143.
S. O. Gollnick and C. M. Brackett, Enhancement of anti-tumor immunity by photodynamic therapy, Immunol. Res., 2009, 46, 216–226.
A. P. Castano, P. Mroz and M. R. Hamblin, Photodynamic therapy and anti-tumour immunity, Nat. Rev. Cancer, 2006, 6, 535–545.
E. Buytaert, M. Dewaele and P. Agostinis, Molecular effectors of multiple cell death pathways initiated by photodynamic therapy, Biochim. Biophys. Acta, 2007, 1776, 86–107.
G. Brouckaert, M. Kalai, D. V. Krysko, X. Saelens, D. Vercammen, M. Ndlovu, G. Haegeman, K. D’Herde and P. Vandenabeele, Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production, Mol. Biol. Cell, 2003, 15, 1089–1100.
M. F. Tsan and B. Gao, Heat shock proteins and immune system, J. Leukocyte Biol., 2009, 85, 905–910.
A. G. Pockley, Heat shock proteins as regulators of the immune response, Lancet, 2003, 362, 469–476.
M. Korbelik, J. Sun and I. Cecic, Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response, Cancer Res., 2005, 65, 1018–1026.
F. Zhou, D. Xing and W. R. Chen, Dynamics andmechanism of HSP70 translocation induced by photodynamic therapy treatment, Cancer Lett., 2008, 264, 135–144.
S. Carta, P. Castellani, L. Delfino, S. Tassi, R. Vene and A. Rubartelli, DAMPs and inflammatory processes: the role of redox in the different outcomes, J. Leukocyte Biol., 2009, 86, 549–555.
G. Minotti, P. Menna, E. Salvatorelli, G. Cairo and L. Gianni, Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity, Pharmacol. Rev., 2004, 56, 185–229.
Y. J. Hsieh, C. C. Wu, C. J. Chang and J. S. Yu, Subcellular localization of Photofrin determines the death phenotype of human epidermoid carcinoma A431 cells triggered by photodynamic therapy:when plasma membranes are the main targets, J. Cell. Physiol., 2003, 194, 363–375.
A. Szokalska, M. Makowski, D. Nowis, G. M. Wilczynski, M. Kujawa, C. Wojcik, I. Mlynarczuk-Bialy, P. Salwa, J. Bil, S. Janowska, P. Agostinis, T. Verfaillie, M. Bugajski, J. Gietka, T. Issat, E. Glodkowska, P. Mrowka, T. Stoklosa, M. R. Hamblin, P. Mroz, M. Jakobisiak and J. Golab, Proteasome inhibition potentiates antitumor effects of photodynamic therapy in mice through induction of endoplasmic reticulum stress and unfolded protein response, Cancer Res., 2009, 69, 4235–4243.
A. D. Garg, T. Verfaillie, N. Rubio, G. B. Ferreira, C. Mathieu and P. Agostinis, Hypericin-PDT treatment of cancer cells leads to surface exposure/extracellular release of DAMPs and activates human immature dendritic cells (abstract), Belg. J.Med. Oncol., 2010, 4, 93–94.
A. Rubartelli and M. T. Lotze, Inside, outside, upside down: damageassociated molecular-pattern molecules (DAMPs) and redox, Trends Immunol., 2007, 28, 429–436.
C. A. Harrison, M. J. Raftery, J. Walsh, P. Alewood, S. E. Iismaa, S. Thliveris and C. L. Geczy, Oxidation regulates the inflammatory properties of the murine S100 protein S100A8, J. Biol. Chem., 1999, 274, 8561–8569.
H. Kazama, J. E. Ricci, J. M. Herndon, G. Hoppe, D. R. Green and T. A. Ferguson, Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein, Immunity, 2008, 29, 21–32.
M. K. Chang, C. J. Binder, M. Torzewski and J. L. Witztum, C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 13043–13048.
E. A. Podrez, E. Poliakov, Z. Shen, R. Zhang, Y. Deng, M. Sun, P. J. Finton, L. Shan, B. Gugiu, P. L. Fox, H. F. Hoff, R. G. Salomon and S. L. Hazen, Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36, J. Biol. Chem., 2002, 277, 38503–38516.
S. Bluml, S. Kirchberger, V. N. Bochkov, G. Kronke, K. Stuhlmeier, O. Majdic, G. J. Zlabinger, W. Knapp, B. R. Binder, J. Stockl and N. Leitinger, Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40, J. Immunol., 2005, 175, 501–508.
A. Furnkranz and N. Leitinger, Regulation of inflammatory responses by oxidized phospholipids: structure-function relationships, Curr. Pharm. Des., 2004, 10, 915–921.
S. L. Hazen, Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity, J. Biol. Chem., 2008, 283, 15527–15531.
M. E. Greenberg, M. Sun, R. Zhang, M. Febbraio, R. Silverstein and S. L. Hazen, Oxidized phosphatidylserine-CD36 interactions play an essential role inmacrophage-dependent phagocytosis of apoptotic cells, J. Exp. Med., 2006, 203, 2613–2625.
D. Nowis, M. Makowski, T. Stoklosa, M. Legat, T. Issat and J. Golab, Direct tumor damage mechanisms of photodynamic therapy, Acta Biochim. Pol., 2005, 52, 339–352.
D. V. Sakharov, E. D. Elstak, B. Chernyak and K. W. Wirtz, Prolonged lipid oxidation after photodynamic treatment. Study with oxidationsensitive probe C11-BODIPY581/591, FEBS Lett., 2005, 579, 1255–1260.
J. Thorburn, H. Horita, J. Redzic, K. Hansen, A. E. Frankel and A. Thorburn, Autophagy regulates selective HMGB1 release in tumor cells that are destined to die, Cell Death Differ., 2008, 16, 175–183.
L. Baricault, J. A. Fransen, M. Garcia, C. Sapin, P. Codogno, L. A. Ginsel and G. Trugnan, Rapid sequestration of DPP IV/CD26 and other cell surface proteins in an autophagic-like compartment in Caco-2 cells treated with forskolin, J. Cell. Sci., 1995, 108(Pt 5), 2109–2121.
D. Tang, R. Kang, K. M. Livesey, C. W. Cheh, A. Farkas, P. Loughran, G. Hoppe, M. E. Bianchi, K. J. Tracey, H. J. Zeh, 3rd and M. T. Lotze, Endogenous HMGB1 regulates autophagy, J. Cell Biol., 2010, 190, 881–892.
M. Dewaele, W. Martinet, N. Rubio, T. Verfaillie, P. A. de Witte, J. Piette and P. Agostinis, Autophagy pathways activated in response to PDT contribute to cell resistance against ROS damage, J. Cell Mol. Med., 2010, DOI: 10.1111/j.1582-4934.2010.01118.x.
M. Dewaele, H. Maes and P. Agostinis, ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy, Autophagy, 2010, 6, DOI: 10.4161/auto.6.7.12113.
J. J. Reiners, Jr., P. Agostinis, K. Berg, N. L. Oleinick and D. Kessel, Assessing autophagy in the context of photodynamic therapy, Autophagy, 2010, 6, 7–18.
G. P. Sims, D. C. Rowe, S. T. Rietdijk, R. Herbst and A. J. Coyle, HMGB1andRAGEin inflammation and cancer, Annu.Rev. Immunol., 2010, 28, 367–388.
H. J. Anders and D. O. Schlondorff, Innate immune receptors and autophagy: implications for autoimmune kidney injury, Kidney Int., 2010, 78, 29–37.
T. Smits, M. M. Kleinpenning, P. E. van Erp, P. C. van de Kerkhof and M. J. Gerritsen, A placebo-controlled randomized study on the clinical effectiveness, immunohistochemical changes and protoporphyrin IX accumulation in fractionated 5-aminolaevulinic acid-photodynamic therapy in patients with psoriasis, Br. J. Dermatol., 2006, 155, 429–436.
L. G. Ratkay, J. D. Waterfield and D. W. Hunt, Photodynamic therapy in immune (non-oncological) disorders: focus on benzoporphyrin derivatives, BioDrugs, 2000, 14, 127–135.
E. Torikai, Y. Kageyama, E. Kohno, T. Hirano, Y. Koide, S. Terakawa and A. Nagano, Photodynamic therapy using talaporfin sodium for synovial membrane from rheumatoid arthritis patients and collageninduced arthritis rats, Clin. Rheumatol., 2007, 27, 751–761.
A. Hansch, O. Frey, M. Gajda, G. Susanna, J. Boettcher, R. Brauer and W. A. Kaiser, Photodynamic treatment as a novel approach in the therapy of arthritic joints, Lasers Surg. Med., 2008, 40, 265–272.
H. Takahashi, S. Komatsu, M. Ibe, A. Ishida-Yamamoto, S. Nakajima, I. Sakata and H. Iizuka, ATX-S10(Na)-PDT shows more potent effect on collagen metabolism of human normal and scleroderma dermal fibroblasts than ALA-PDT, Arch. Dermatol. Res., 2006, 298, 257–263.
A. Olejek, K. Steplewska, A. Gabriel, I. Kozak-Darmas, A. Jarek, S. Kellas-Sleczka, F. Bydlinski, K. Sieron-Stoltny, S. Horak, A. Chelmicki and A. Sieron, Efficacy of Photodynamic Therapy in Vulvar Lichen Sclerosus Treatment Based on Immunohistochemical Analysis of CD34, CD44, Myelin Basic Protein, and Ki67 Antibodies, Int. J. Gynecol. Cancer, 2010, 20, 879–887.
S. Motta and M. Monti, Photodynamic therapy-a promising treatment option for autoimmune skin ulcers: a case report, Photochem. Photobiol. Sci., 2007, 6, 1150–1151.
M. Subbarayan, U. O. Hafeli, D. K. Feyes, J. Unnithan, S. N. Emancipator and H. Mukhtar, A simplified method for preparation of 99mTc-annexin V and its biologic evaluation for in vivo imaging of apoptosis after photodynamic therapy, J. Nucl. Med., 2003, 44, 650–656.
S. Suzuki and A. B. Kulkarni, Extracellular heat shock protein HSP90beta secreted by MG63 osteosarcoma cells inhibits activation of latent TGF-beta1, Biochem. Biophys. Res. Commun., 2010, 398, 525–531.
J. R. Riddell, X. Y. Wang, H. Minderman and S. O. Gollnick, Peroxiredoxin 1 stimulates secretion of proinflammatory cytokines by binding to TLR4, J. Immunol., 2009, 184, 1022–1030.
A. Iwata, V. Morgan-Stevenson, B. Schwartz, L. Liu, J. Tupper, X. Zhu, J. Harlan and R. Winn, Extracellular BCL2 proteins are dangerassociated molecular patterns that reduce tissue damage in murine models of ischemia-reperfusion injury, PLoS One, 2010, 5, e9103.
K. E. Blume, S. Soeroes, M. Waibel, H. Keppeler, S. Wesselborg, M. Herrmann, K. Schulze-Osthoff and K. Lauber, Cell surface externalization of annexin A1 as a failsafe mechanism preventing inflammatory responses during secondary necrosis, J. Immunol., 2009, 183, 8138–8147.
A. A. Manfredi and P. Rovere-Querini, The mitochondrion–a Trojan horse that kicks off inflammation?, N. Engl. J. Med., 2010, 362, 2132–2134.
Q. Zhang, M. Raoof, Y. Chen, Y. Sumi, T. Sursal, W. Junger, K. Brohi, K. Itagaki and C. J. Hauser, Circulating mitochondrial DAMPs cause inflammatory responses to injury, Nature, 2010, 464, 104–107.
A. Babelova, K. Moreth, W. Tsalastra-Greul, J. Zeng-Brouwers, O. Eickelberg, M. F. Young, P. Bruckner, J. Pfeilschifter, R. M. Schaefer, H. J. Grone and L. Schaefer, Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors, J. Biol. Chem., 2009, 284, 24035–24048.
L. Schaefer, Extracellularmatrix molecules: endogenous danger signals as new drug targets in kidney diseases, Curr. Opin. Pharmacol., 2010, 10, 185–190.
F. G. Goh, A. M. Piccinini, T. Krausgruber, I. A. Udalova and K. S. Midwood, Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation, J. Immunol., 2010, 184, 2655–2662.
T. E. Ichim, Z. Zhong, S. Kaushal, X. Zheng, X. Ren, X. Hao, J. A. Joyce, H. H. Hanley, N. H. Riordan, J. Koropatnick, V. Bogin, B. R. Minev, W. P. Min and R. H. Tullis, Exosomes as a tumor immune escape mechanism: possible therapeutic implications, J. Transl. Med., 2008, 6, 37.
L. Zitvogel, A. Tesniere and G. Kroemer, Cancer despite immunosurveillance: immunoselection and immunosubversion, Nat. Rev. Immunol., 2006, 6, 715–727.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of a themed issue on immunological aspects and drug delivery technologies in PDT.
Rights and permissions
About this article
Cite this article
Garg, A.D., Krysko, D.V., Vandenabeele, P. et al. DAMPs and PDT-mediated photo-oxidative stress: exploring the unknown. Photochem Photobiol Sci 10, 670–680 (2011). https://doi.org/10.1039/c0pp00294a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c0pp00294a