Abstract
A re-examination of former concepts is required to meet today’s medical challenges in allergic rhinitis. Previously, neutrophils have been treated as a relatively homogenous cell population found in the nose both when the patient is suffering at the height of the allergic season as well as when the patient report no symptoms. However, new data indicates that neutrophils can be divided into different subsets with diverse roles in inflammation. We showed increased levels of neutrophils in peripheral blood, nasal biopsies and nasal lavage fluid (NAL) from allergic patients during the pollen season compared to healthy controls. A closer examination revealed that the activated subset of neutrophils, CD16high CD62Ldim, outweighed the normal form CD16high CD62Lhigh in nasal tissue among these patients. This skewed distribution was not seen in controls. The normal subset prevailed in peripheral blood from patients as well as controls, whereas CD16high CD62Ldim and CD16dim CD62Ldim subsets, the latter considered “end state” neutrophils before apoptosis, were elevated in NAL. Functional in vitro experiments revealed that activated neutrophils exhibit a T cell priming capacity and an ability to enhance eosinophil migration. Activated neutrophils may thus contribute to allergic inflammation seen in allergic rhinitis by priming T cells and attracting eosinophils.
Similar content being viewed by others
Introduction
Allergic rhinitis (AR) is an inflammatory disease of the nasal mucosa, induced by a reaction to a normally harmless antigen. It is characterized by troublesome local nasal symptoms as well as general fatigue, negatively impacting quality of life. In addition, the significant prevalence of AR is associated with staggering indirect costs for the Western societies1,2,3. In order to develop novel strategies for further treatment, traditional concepts of allergic inflammation have to be re-examined4.
Eosinophils are well described markers of the allergic reaction, known to play an important role in Th2-mediated immune responses5,6,7,8,9. Neutrophils have evoked less interest, as they are found in the nose both when the patient is suffering at the height of the allergy season as well as when no symptoms can be reported. Nevertheless the number of neutrophils increases in the nose in symptomatic individuals during the allergy season and their large absolute cell number in comparison with corresponding eosinophils deserves attention10,11,12.
Neutrophils have long been considered short-lived, terminally differentiated cells with a well-established role in acute bacterial infections13,14. However, information has recently emerged indicating that neutrophils can be divided into different subsets and that the various subsets might have diverse roles in inflammatory diseases. The new subsets are defined by differences in expression of FcγRIII (CD16) and L-selectin (CD62L). Three different subsets have recently been identified: CD16dim CD62Lhigh (immature), CD16high CD62Lhigh (mature) and CD16high CD62Ldim (activated and CD11bbright as well as CD11cbright)15. The same authors reported that the activated subset of mature human neutrophils causes certain immunological responses. Differentiated, CD11b+ neutrophils have further been suggested to be reduced at the site of C. perfringens infections through systemic reduction of mature neutrophils, possibly explaining polymicrobial infections seen in these patients16.
We now suggest that some of these neutrophil subsets might affect the course of inflammation seen in AR. Hence, the present study is designed to identify these subsets as well as determine their ability to traffic between different compartments i.e. peripheral blood, nasal mucosa and nasal lavage fluid (NAL) during the allergic season. Further, the relation between activated neutrophils and CD4+ T cells, as well as eosinophils are explored in vitro under allergic inflammatory conditions17,18,19,20,21,22.
Results
Neutrophils increase in allergy
The first set of experiments investigated the anticipated change in neutrophil number in peripheral blood, nasal biopsies and NAL caused by the pollen season. Neutrophil numbers were found to be enhanced in peripheral blood from patients with AR compared to healthy controls (p < 0.0013) (Fig. 1(a)). Correspondingly, the neutrophil fraction was also significantly increased in nasal biopsies (p < 0.0013) and NAL (p < 0.0127) from patients with AR (Fig. 1(b,c)).
(a) Fraction of neutrophils in blood, (b) nasal biopsies and (c) NAL from patients with AR during pollen season (n = 8) and healthy controls (n = 6). *Indicates statistical significance (p < 0.05), **indicates statistical significance (p < 0.01) (unpaired t-tests (Mann-Whitney)). Data are shown as mean ± S.E.M.
Different subsets increase in different compartments
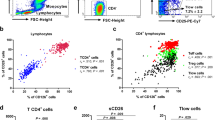
In order to further explore the increase in neutrophils in AR patients, the cells were categorised by their expression of CD16 and CD62L (Fig. 2(a)). The phenotypically mature neutrophil subset CD16high CD62Lhigh dominated in peripheral blood. In nasal biopsies, both the mature neutrophil subset, CD16high CD62Lhigh, and the activated form CD16high CD62Ldim, with a hypersegmented nuclear morphology, were elevated (Fig. 2(a)). Finally, the subsets CD16high CD62Ldim and CD16dim CD62Ldim, considered the end state before apoptosis, were elevated in NAL. Healthy controls exhibited the same general compartmental subset pattern as the AR patients with more differentiated subsets in nasal biopsies and even more in NAL compared to in peripheral blood (Fig. 2(b)). It is important to notice that the activated neutrophil subset, CD16high CD62Ldim, was elevated compared to the mature neutrophil subset CD16high CD62Lhigh in biopsies from patients with AR (p < 0.041) (Fig. 2(c)). This was not seen in healthy controls.
(a) The distribution of neutrophil subsets in blood (n = 7), nasal biopsies (n = 7) and NAL (n = 5) in AR-patients during pollen season and (b) in healthy controls (n = 6). (c) Subset distribution in nasal biopsies. (d) NAL incubated with Abs against CD16 and CD62L demonstrating the subset CD16dim CD62Ldim. Gating procedures of blood and NAL incubated with (e) eosinophilic Abs and (f) viability experiments. *Indicates statistical significance (p < 0.05), **indicates statistical significance (p < 0.01), ***indicates statistical significance (p < 0.001) (two-way ANOVA with Bonferroni post-test). Data are shown as (a-b) mean ± S.E.M. or (c) mean.
The subset CD16dim CD62Ldim has previously not been described (Fig. 2(d)). To ensure that these cells are neutrophils, antibodies were used to exclude eosinophils (Fig. 2(e)). In addition, the majority of cells from peripheral blood and NAL were alive since they were negative for the viability dye Zombie (Fig. 2(f)).
Activated neutrophils can help activate T cells
Functional in vitro experiments were performed to evaluate the immunological importance of the activated neutrophil subsets. Mature neutrophils, CD16high CD62Lhigh (Fig. 3(a)), were isolated from peripheral blood from healthy controls and activated in vitro with LPS, TNF-α and IL-8. Flow cytometric analysis verified an increase of the activated neutrophil subset (CD16high CD62Ldim) after activation (Fig. 3(b)). The expression of the activation marker CD66b increased simultaneously on neutrophils, supporting their state of activation (see Supplementary Fig. S1). A schematic figure illustrates the phenotypic maturation of neutrophils in the activation process (Fig. 3(c)). After thoroughly washing the neutrophils, isolated autologous T cells from peripheral blood were added in a co-culture. There was an increase in both the fraction of CD69+/CD4+ T cells (p < 0.0007) (Fig. 4(a)) and in the expression levels of CD69 on CD4+ T cells (p < 0.0051) (Fig. 4(b)) when T cells had been primed with activated neutrophils prior to CD3 stimulation. The same findings were made when using blood from patients with AR outside the pollen season (see Supplementary Fig. S2(a,b)). The increased activation of CD4+ T cells was confined to CD45RO− T cells indicating that neutrophils specifically affected naïve CD4+ T cells (see Supplementary Fig. S3). The T cell activation level in control experiments with naïve neutrophils was comparable with controls using only T cells and CD3, ruling out a T cell priming capacity for naïve neutrophils. Experiments with transwell cell cultures were set up to determine how T cell priming was mediated by activated neutrophils. When using transwell plates, no increase in CD69+/CD4+ T cells was seen upon priming with activated neutrophils (Fig. 4(c,d)) indicating that the enhanced T cell activation of neutrophils was cell-cell contact or close contact dependent. As a control, monocytes were analysed and no increased activation was seen, making it unlikely that the facilitated T cell activation was mediated by impurities from activated monocytes (see Supplementary Fig. S4). Representative dot plots illustrates the gating procedures (Fig. 4(e)).
The (a) fraction and (b) MFI of CD69+/CD4+ T cells primed with naïve and activated neutrophils. (c,d) Experiments with and without transwell plates. Control = no added neutrophils. (e) Representative dot plots illustrating gating procedures. *Indicates statistical significance (p < 0.05), **indicates statistical significance (p < 0.01), ***indicates statistical significance (p < 0.001) (non-parametric (Friedman test) one-way ANOVA with Dunn’s post-test). Data are shown as mean ± S.E.M.
Activated neutrophils mediate eosinophil migration
To further analyse the importance of neutrophils in allergic inflammation, experiments were set up to study the impact of activated neutrophils on eosinophils. Isolated neutrophils activated in vitro with LPS, TNF-α and IL-8 upregulated eosinophilic migration after 3 h of incubation in the transwell system (Fig. 5(a)). Naïve neutrophils had no impact on eosinophil migration. Thus, we concluded that activated neutrophils can increase eosinophil migration, potentially accounting for the local eosinophil infiltration seen in allergy. Representative dot plots illustrates the gating procedures (Fig. 5(b)).
Discussion
Patients with AR exhibited increased numbers of neutrophils in peripheral blood, nasal biopsies and NAL during the pollen season. When studying this amplification on a subset-level, it was evident that the mature neutrophil subset CD16high CD62Lhigh dominated in peripheral blood while the subsets CD16high CD62Lhigh together with the activated form CD16high CD62Ldim were elevated in nasal biopsies. The activated subset CD16high CD62Ldim fraction was significantly higher than the normal fraction in nasal tissue from AR-patients. This was not convincingly seen in the healthy controls. Finally, the subsets CD16high CD62Ldim and CD16dim CD62Ldim, considered the end state before apoptosis, were elevated in NAL. A T cell priming capacity of activated neutrophils was demonstrated in vitro by co-culturing activated neutrophils and CD4+ T cells. Since no such priming was seen using a transwell system, this interaction was likely mediated by close or direct cell-cell contact. Further, activated neutrophils were found to upregulate eosinophil migration.
Special attention has lately been paid to the findings that neutrophil subsets can be defined by differences in CD16 and CD62L expression15,23. The CD16high CD62Ldim neutrophil subset has previously been suggested to cause T cell inhibition through Mac-1 resulting in immunosuppression15. However, the way in which different neutrophil subsets influence various diseases has scarcely been investigated. We have previously demonstrated that activated neutrophils seem to have anti-tumorigenic properties24. Moreover, we have suggested that the enhanced systemic adaptive immune response seen among patients with AR might protect against head and neck squamous cell carcinoma, a phenomenon likely mediated by peripheral blood mononuclear cells25. The same subtype has been demonstrated in other cancer forms26. Neutrophils from allergic patients have been shown to downregulate surface expression of CD62L upon allergen stimulation27. The distribution of different neutrophils based on their expression of CD16 and CD62L has to our knowledge however never been studied in allergy.
Both neutrophils and eosinophils are known to increase upon allergen exposure. Even though the neutrophils far outnumber the amount of eosinophils found both locally and systemically during symptomatic AR, the former has been given much less attention as potential players in the allergic reaction (Fig. 1). This is probably due to the fact that neutrophils can be found in the nose in symptomatic patients at the height of the allergic season as well as in the asymptomatic phase. However, when examining changes in neutrophil subsets in different compartments, a different picture emerges (Fig. 2). It becomes clear that activated and differentiated neutrophil subsets accumulate at the site of allergic provocation, in this case in the nose. Furthermore, we demonstrated that these activated neutrophils have the ability to prime CD4+ T cells, a phenomenon known to be of great importance for the start of allergic inflammation at the site of antigen exposure28,29. This notion is supported by a recent publication demonstrating that timothy grass pollen can stimulate neutrophil immune responses through the secretion of IL-811. In addition, the T cells in our experiment were CD45RO− negative, indicating that the activated neutrophil subset could have a role in the allergic sensitisation process, by affecting naïve antigen-specific CD4+ T cells30. Hence, locally activated neutrophils in the upper airways seem to have the ability to mediate the inflammatory process in allergy. Further, neutrophils have recently been shown to be components of the response to, immunisation accumulating in lymph nodes and forming an adaptive immune response31. Neutrophil subsets could even partly explain the airway epithelial injury seen in asthma32,33. Altogether, these reports suggest that neutrophils are more complex than previously thought.
T cell activation and proliferation is central in allergic immunity. However, it is still not completely understood which cells are responsible for local mucosal T cell activation. It has previously been shown that it takes several days to recruit conventional dendritic cells to the nasal mucosa upon allergen challenge34. We have earlier shown the immunological importance of nasal epithelial cells activating T cell at the site of allergen exposure, i.e. the nose35. In addition, previous publications in mice have showed that neutrophils can influence CD8+ T cell response and possibly also direct or prime Th1 T cell responses36,37,38. The current experiments demonstrate that activated human neutrophils can prime T cells, thus facilitating CD3 activation. No such priming was seen when naïve neutrophils were used or when T cells and CD3 were used alone speaking against potential contamination from monocytes. Separate analyses of monocytes in wells containing activated neutrophils demonstrated no monocyte activation, definitively ruling out a role for monocytes in facilitating T cell activation (see Supplementary Fig. S4). These assays identify that activated neutrophils have stimulatory properties on T cells, further suggesting the possible functional impact of locally activated neutrophil subsets in allergic patients.
The presence of both circulating and local eosinophils during ongoing allergic inflammation is well established39. In contrast, the detailing of their pharmacological suppression and the resulting clinical improvement has over the years been limited40,41. Interestingly, a significant decrease of not only eosinophils but also neutrophils in the nasal mucosa was seen upon sublingual immunotherapy (IT) to Parietaria species42. Neutrophils were also shown to be decreased in a skin chamber model upon AR IT43,44. Further, IL-9 positive neutrophils increased in the nasal mucosa during pollen season, something that could be successfully inhibited by IT45.
The present data on neutrophil subsets might be a next step in understanding the great infiltration of neutrophils in the nasal mucosa in allergy. We demonstrate the ability for activated subsets to directly prime T cells and enhance migration of eosinophils46,47. This is in line with previous publications suggesting a more complex role for neutrophils28,29,31,46. Further experimental studies depleting or deactivating the activated neutrophils in an allergic setting, potentially affecting the course of the inflammation, are required. This could open up new therapeutic possibilities for AR.
Methods
Patients
Eight non-smoking AR patients with allergy to birch pollen (n = 3), grass pollen (n = 1) or both (n = 4) were included in the study (mean age 28 years; M:F 2:6). All patients were healthy with the exception of their allergy. Their allergy was diagnosed on the basis of clinical history and positive skin prick test (SPT) and/or the radioallergosorbent test (RAST) for allergen-specific IgE. Samples were taken in-season and all patients had nasal symptoms of allergy at the time of participation. Seven patients had been off antihistamine treatment for more than 48 h, one had been off antihistamine treatment for more than 24 h. Four patients reported no use of topical nasal steroids for at least a month. Four patients had been off topical nasal steroids for more than three days. No patient had been on inhaled steroids for at least one month. In addition, six healthy non-smoking controls were included (mean age 21 years; M:F 2:4). RAST for allergen-specific IgE were negative for all healthy controls included in the study. The patients and healthy controls reported no history of infections in the airways or in the rest of the body three weeks prior to participating. The study was approved by the local ethical committee in Stockholm, Karolinska Institutet, and all patients and healthy controls gave their written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Human nasal biopsies
Nasal biopsies were taken from inferior turbinates of healthy non-smoking controls under local anaesthesia as previously described48. Tissue was put through a 100 μm cell strainer (BD Falcon, Franklin Lakes, NJ, USA), into Dulbecco’s Modified Eagle’s Medium and Nutrient Mixture Ham’s F12 Medium (DMEM/F-12) (Gibco®, Paisley, UK) containing 10% FBS and incubated at RT for 5 min. Cells were washed and centrifuged and the pellet was resuspended in PBS containing 2% FBS prior to analysis with flow cytometry.
Human peripheral blood
Peripheral blood was collected in heparin tubes, lysed with formic acid and ion solution, and finally stained with different antibodies for flow cytometry (see Antibodies and flow cytometry section for details).
Recovery of nasal lavage fluid
Nasal lavage fluid was collected as previously described48. In brief, after cleaning excess mucous by forceful exsufflation, 8–10 ml of sterile saline solution (0.9% NaCl) at RT was aerosolised into the nostrils and passively collected from the nostrils. When 7 ml of fluid were recovered into a graded test tube, it was centrifuged for 10 min. The pellet was resuspended in PBS containing 2% FBS before analysis with flow cytometry.
T cell stimulation
For all in vitro experiments, healthy controls with no known allergies were recruited. For the T cell stimulation experiments, patients with birch and/or grass pollen allergy were also recruited. All participants were non-smokers and all patients were healthy with the exception of their allergy. Blood was collected in heparin tubes. T cells and neutrophils were isolated from peripheral blood using ficoll-paque (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. The erythrocytes in the granulocyte rich pellet were lysed (0.8% NH4Cl, 10 mM KHCO3 and 0.1 mM EDTA). Neutrophils were further purified with CD15 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. The neutrophils were diluted to a concentration of 2 × 106cells/ml in TexMACS Medium (Miltenyi Biotec) containing 10% autologous plasma, 100 U/mL penicillin and 100 μg/mL streptomycin (Life Technologies, Eugene, OR, USA). Thereafter, the neutrophils were activated with 1 μg/ml LPS (product no: L2654, Sigma-Aldrich, St. Louis, MO, USA), 5 ng/ml TNF-α and 10 ng/ml IL-8 (R&D System, Minneapolis, MN, USA) for 15 min and then washed with PBS twice. The lymphocyte interface from the ficoll-paque isolation step was collected and washed with PBS. The cells were diluted to a concentration of 2 × 106cells/ml in the same medium as the neutrophils.
A co-culture with activated neutrophils (5 × 105) and lymphocytes (5 × 105) was set up in wells for 30 min. Thereafter, CD3 (0.05 μg) (clone OKT3, BioLegend®, San Diego, CA, USA) was added and incubated for 90 min, for T cell activation. The cell suspension was then collected and analysed with flow cytometry.
For blocking cell-cell contact, transwell plates (0.4 μm, Corning, NY, USA) were used in separate experiments to separate neutrophils from lymphocytes. In these experiments, CD3 was added to the lymphocyte part of the transwell plates.
Control experiments with naïve neutrophils were run in all experiments.
Eosinophil migration experiment
Eosinophils and neutrophils were collected from whole blood using MACSxpress Neutrophil Isolation Kit, human and Eosinophil Isolation Kit, human, (Miltenyi Biotec) according to the manufacturer’s instructions. Remaining erythrocytes were lysed. Cells were diluted in Complete Medium (CM) consisting of RPMI 1640 (Invivogen, San Diego, CA, USA) with 10% autologous plasma, penicillin 100 U/mL and streptomycin 100 μg/mL (Invitrogen, Carlsband, CA, USA), to a concentration of 1 × 105cells/ml. Migration set up was conducted with transwell plates (3.0 μm PTFE Collagen Coated Membrane, Corning, NY, USA). Cells were added (5 × 105 neutrophils in the bottom well and 2.5 × 105 eosinophils in the insert) and incubated for 3 h at 37 °C in a humidified 5% CO2 air atmosphere. Inserts were removed and cell suspension in the wells were analysed with flow cytometry using CountBright TM Absolute Counting Beads (Life Technologies, Eugene, OR, USA).
Antibodies and flow cytometry
The following monoclonal antibodies were purchased from BD Biosciences (San Jose, CA, USA): anti-human CD4 (clone RPA-T4), CD8 (clone RPA-T8), CD5 (clone UCHT2), CD11b (clone ICRF44), CD14 (clone M5E2), CD15 (clone W6D3), CD16 (clone 3G8), CD45 (clone 2D1), CD45RA (clone HI100), CD45RO (clone UCHL1), CD56 (clone B159), CD62L (clone DREG-56), CD69 (clone FN50), CD80 (clone L307,4 and BB1), CD86 (clone 2331), CCR3 (clone 5E8) and CDw125 (clone A14). Anti-human HLA-DR (clone L243), CD43 (clone MEM-59) and SIGLEC-8 (clone 7C9) were purchased from BioLegend® (San Diego, CA, USA). Anti-human ICAM-1 (clone BBIG-I1) was purchased from R&D (Minneapolis, MN, USA) and Zombie NIR™ Fixable Viability Kit was purchased from BioLegend®. All antibodies were titrated for optimum concentration before use, with dilutions ranging from 1:5 to 1:400. Cells were identified based on forward and side scatter properties. In flow cytometry assays studying CD69, fluorescence minus one (FMO) controls were used. Cells were analysed on an LRSFortessa analyser (BD Biosciences) and data were processed using FlowJo software (©Tree Star, Inc., Ashland, USA).
Statistics
Statistical differences between patients with allergy and healthy controls were performed using unpaired t-tests (Mann-Whitney). For more than two sets of matched data, a two-way ANOVA with Bonferroni post-tests was performed. For the in vitro experiments, a non-parametric (Friedman test) one-way ANOVA was used together with Dunn’s post-test, except for the eosinophil migration experiments where Tukey’s multiple comparisons test was used. A p-value of 0.05 or less was considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001). Statistical analyses were performed using GraphPad Prism software (version 6.0, GraphPad Software, La Jolla, CA). All data are shown as mean ± S.E.M. For human data, n equals the number of patients.
Additional Information
How to cite this article: Arebro, J. et al. A possible role for neutrophils in allergic rhinitis revealed after cellular subclassification. Sci. Rep. 7, 43568; doi: 10.1038/srep43568 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bousquet, J. et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 63 Suppl 86, 8–160, doi: 10.1111/j.1398-9995.2007.01620.x (2008).
Hellgren, J., Cervin, A., Nordling, S., Bergman, A. & Cardell, L. O. Allergic rhinitis and the common cold–high cost to society. Allergy 65, 776–783, doi: 10.1111/j.1398-9995.2009.02269.x (2010).
Cardell, L. O. et al. TOTALL: high cost of allergic rhinitis-a national Swedish population-based questionnaire study. NPJ primary care respiratory medicine 26, 15082, doi: 10.1038/npjpcrm.2015.82 (2016).
Steinsvaag, S. K. Allergic rhinitis: an updated overview. Current allergy and asthma reports 12, 99–103, doi: 10.1007/s11882-012-0242-y (2012).
Blanchard, C. & Rothenberg, M. E. Biology of the eosinophil. Advances in immunology 101, 81–121, doi: 10.1016/s0065-2776(08)01003-1 (2009).
Broide, D. & Sriramarao, P. Eosinophil trafficking to sites of allergic inflammation. Immunological reviews 179, 163–172 (2001).
Hogan, S. P. et al. Eosinophils: biological properties and role in health and disease. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 38, 709–750, doi: 10.1111/j.1365-2222.2008.02958.x (2008).
Kita, H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunological reviews 242, 161–177, doi: 10.1111/j.1600-065X.2011.01026.x (2011).
Trivedi, S. G. & Lloyd, C. M. Eosinophils in the pathogenesis of allergic airways disease. Cell Mol Life Sci 64, 1269–1289, doi: 10.1007/s00018-007-6527-y (2007).
Fransson, M., Benson, M., Wennergren, G. & Cardell, L. O. A role for neutrophils in intermittent allergic rhinitis. Acta Otolaryngol 124, 616–620 (2004).
Hosoki, K., Boldogh, I. & Sur, S. Innate responses to pollen allergens. Current opinion in allergy and clinical immunology 15, 79–88, doi: 10.1097/aci.0000000000000136 (2015).
Varney, V. A. et al. Immunohistology of the nasal mucosa following allergen-induced rhinitis. Identification of activated T lymphocytes, eosinophils, and neutrophils. Am Rev Respir Dis 146, 170–176 (1992).
Jaillon, S. et al. Neutrophils in innate and adaptive immunity. Seminars in immunopathology 35, 377–394, doi: 10.1007/s00281-013-0374-8 (2013).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nature reviews. Immunology 13, 159–175, doi: 10.1038/nri3399 (2013).
Pillay, J. et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest 122, 327–336 (2012).
Takehara, M. et al. Clostridium perfringens alpha-Toxin Impairs Innate Immunity via Inhibition of Neutrophil Differentiation. Scientific reports 6, 28192, doi: 10.1038/srep28192 (2016).
Puljic, R. et al. Lipopolysaccharide-induced lung inflammation is inhibited by neutralization of GM-CSF. European journal of pharmacology 557, 230–235, doi: 10.1016/j.ejphar.2006.11.023 (2007).
Bachar, O., Gustafsson, J., Jansson, L., Adner, M. & Cardell, L. O. Lipopolysaccharide administration to the allergic nose contributes to lower airway inflammation. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 37, 1773–1780, doi: 10.1111/j.1365-2222.2007.02842.x (2007).
Bradding, P. et al. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. American journal of respiratory cell and molecular biology 10, 471–480, doi: 10.1165/ajrcmb.10.5.8179909 (1994).
Abdelaziz, M. M. et al. The effect of conditioned medium from cultured human bronchial epithelial cells on eosinophil and neutrophil chemotaxis and adherence in vitro . American journal of respiratory cell and molecular biology 13, 728–737, doi: 10.1165/ajrcmb.13.6.7576711 (1995).
Thomas, P. S. Tumour necrosis factor-alpha: the role of this multifunctional cytokine in asthma. Immunology and cell biology 79, 132–140, doi: 10.1046/j.1440-1711.2001.00980.x (2001).
Gibson, P. G., Simpson, J. L. & Saltos, N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 119, 1329–1336 (2001).
Kamp, V. M. et al. Human suppressive neutrophils CD16bright/CD62Ldim exhibit decreased adhesion. J Leukoc Biol 92, 1011–1020 (2012).
Millrud, C. R. et al. The activation pattern of blood leukocytes in head and neck squamous cell carcinoma is correlated to survival. PLoS One 7, 10 (2012).
Millrud, C. R. et al. Inverse immunological responses induced by allergic rhinitis and head and neck squamous cell carcinoma. PLoS One 9 (2014).
Hao, S., Andersen, M. & Yu, H. Detection of immune suppressive neutrophils in peripheral blood samples of cancer patients. Am J Blood Res 3, 239–245 (2013).
Monteseirin, J. et al. L-selectin expression on neutrophils from allergic patients. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 35, 1204–1213, doi: 10.1111/j.1365-2222.2005.02320.x (2005).
Matsumoto, M. et al. CD43 functions as a ligand for E-Selectin on activated T cells. J Immunol 175, 8042–8050 (2005).
Zhang, J. et al. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J Immunol 169, 2236–2240 (2002).
Kinnunen, T. et al. Allergen-specific naive and memory CD4+ T cells exhibit functional and phenotypic differences between individuals with or without allergy. European journal of immunology 40, 2460–2469, doi: 10.1002/eji.201040328 (2010).
Desbien, A. L. et al. Squalene emulsion potentiates the adjuvant activity of the TLR4 agonist, GLA, via inflammatory caspases, IL-18, and IFN-gamma. European journal of immunology 45, 407–417, doi: 10.1002/eji.201444543 (2015).
Mabalirajan, U. et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Scientific reports 3, 1349, doi: 10.1038/srep01349 (2013).
Wang, S. Z. et al. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. The European respiratory journal 12, 612–618 (1998).
Eguiluz-Gracia, I. et al. Rapid recruitment of CD14 monocytes in experimentally induced allergic rhinitis in human subjects. The Journal of allergy and clinical immunology, doi: 10.1016/j.jaci.2015.11.025 (2016).
Arebro, J. et al. Antigen-presenting epithelial cells can play a pivotal role in airway allergy. The Journal of allergy and clinical immunology 137, 957–960, e957, doi: 10.1016/j.jaci.2015.08.053 (2016).
Beauvillain, C. et al. Neutrophils efficiently cross-prime naive T cells in vivo . Blood 110, 2965–2973, doi: 10.1182/blood-2006-12-063826 (2007).
Maletto, B. A. et al. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood 108, 3094–3102, doi: 10.1182/blood-2006-04-016659 (2006).
Abi Abdallah, D. S., Egan, C. E., Butcher, B. A. & Denkers, E. Y. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. International immunology 23, 317–326, doi: 10.1093/intimm/dxr007 (2011).
Bentley, A. M. et al. Immunohistology of the nasal mucosa in seasonal allergic rhinitis: increases in activated eosinophils and epithelial mast cells. The Journal of allergy and clinical immunology 89, 877–883 (1992).
Flood-Page, P. T., Menzies-Gow, A. N., Kay, A. B. & Robinson, D. S. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. American journal of respiratory and critical care medicine 167, 199–204, doi: 10.1164/rccm.200208-789OC (2003).
Liu, Y., Zhang, S., Li, D. W. & Jiang, S. J. Efficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trials. PLoS One 8, e59872, doi: 10.1371/journal.pone.0059872 (2013).
Passalacqua, G. et al. Clinical and immunologic effects of a rush sublingual immunotherapy to Parietaria species: A double-blind, placebo-controlled trial. The Journal of allergy and clinical immunology 104, 964–968 (1999).
Nish, W. A. et al. The effect of immunotherapy on the cutaneous late phase response to antigen. The Journal of allergy and clinical immunology 93, 484–493 (1994).
Shamji, M. H. & Durham, S. R. Mechanisms of immunotherapy to aeroallergens. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 41, 1235–1246, doi: 10.1111/j.1365-2222.2011.03804.x (2011).
Nouri-Aria, K. T., Pilette, C., Jacobson, M. R., Watanabe, H. & Durham, S. R. IL-9 and c-Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: effect of immunotherapy. The Journal of allergy and clinical immunology 116, 73–79, doi: 10.1016/j.jaci.2005.03.011 (2005).
Alam, R. & Busse, W. W. The eosinophil–quo vadis? The Journal of allergy and clinical immunology 113, 38–42, doi: 10.1016/j.jaci.2003.10.054 (2004).
Kikuchi, I. et al. Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils. American journal of respiratory cell and molecular biology 34, 760–765, doi: 10.1165/rcmb.2005-0303OC (2006).
Fransson, M. et al. Expression of Toll-like receptor 9 in nose, peripheral blood and bone marrow during symptomatic allergic rhinitis. Respiratory research 8, 17, doi: 10.1186/1465-9921-8-17 (2007).
Acknowledgements
We thank Ronia Razavi for laboratory work, the nurses Maria Axelsson, Carina Israelsson and Eva Fransson for assisting the handling of patients and Olivia Larsson for proof reading. The main author thanks Professor Jay Nadel, UCSF for introducing him to the beauty of neutrophils. This work was supported by grants from the Swedish Research Council, Karolinska Institutet, Karolinska University Hospital, the Hesselman’s Research Foundation, the Acta Oto-Laryngologica Foundation and Nachmanssons Foundation.
Author information
Authors and Affiliations
Contributions
Conception and design and analysis and interpretation of data: J.A., S.E., E.H., O.W., S.K.G. and L.-O.C. Providing samples: mainly J.A. Performance of experiments: mainly S.E. and E.H. Manuscript writing: J.A. Critical revision of the article for important intellectual content: J.A., S.E., E.H., O.W., S.K.G. and L.-O.C. The manuscript was finally approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Arebro, J., Ekstedt, S., Hjalmarsson, E. et al. A possible role for neutrophils in allergic rhinitis revealed after cellular subclassification. Sci Rep 7, 43568 (2017). https://doi.org/10.1038/srep43568
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43568
This article is cited by
-
Upregulated expression of Notch1/4 - JAG-1/DLL-1 detected in allergic rhinitis
Allergy, Asthma & Clinical Immunology (2023)
-
An Emerging Role of Extracellular Traps in Chronic Rhinosinusitis
Current Allergy and Asthma Reports (2023)
-
Neutrophil infiltrates and eosinophil aggregates in chronic rhinosinusitis with nasal polyps and EGPA
Clinical Rheumatology (2021)
-
The impact of allergen exposure and specific immunotherapy on circulating blood cells in allergic rhinitis
World Allergy Organization Journal (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.