Abstract
In recent years, there is a growing interest towards the green synthesis of metal nanoparticles, particularly from plants; however, yet no published study on the synthesis of ZnO.NPs using the Deverra tortuosa extract. Through this study, zinc oxide nanoparticles (ZnO.NPs) have been synthesized based on using the environmentally benign extract of the aerial parts of D. tortuosa as a reducing and capping agent. ZnO.NPs synthesis was confirmed using UV-Visible (UV-Vis) spectroscopy, Fourier Transform Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD) and High Resolution-Transmission Electron Microscope (HR-TEM). The qualitative and quantitative analyses of plant extract were done. The potential anticancer activity was in vitro investigated against two cancer cell lines (human colon adenocarcinoma “Caco-2” and human lung adenocarcinoma “A549”) compared to their activities on the human lung fibroblast cell line (WI38) using the MTT assay. Both the aqueous extract and ZnO.NPs showed a remarkable selective cytotoxicity against the two examined cancer cell lines.
Similar content being viewed by others
Introduction
Nowadays, nanotechnology is expected to be the basis of many biotechnological innovations in the 21st century and regarded as the upcoming industrial revolution. Nanomaterials have been called ‘a wonder of modern medicine’ and elicited much interest over the past few decades1. Nanomaterials are of great importance because of their superior physicochemical and biological properties over their bulk phase. The size of these nanostructured materials (1–100 nm) offers a higher surface to volume ratio which led to high surface reactivity2. This distinct property allowed them to be utilized in vast applications in many fields ranging from material science to biotechnology. Nanobiotechnology is the merge between biotechnology and nanotechnology for developing biosynthetic and ecofriendly technology for the synthesis of nanomaterials3.
Progress in utilizing inorganic nanoparticles for biomedical applications taking into the account the environmental aspect stimulated the need to synthesize them using the green chemistry strategies via biological systems4,5. Various studies suggested that plants seem to be the superior candidate and are proper for large scale biosynthesis of nanoparticles where the rate of synthesis is faster than that in the case of other organisms. In addition, the nanoparticles produced through plants are more various in shape and size in comparison with those produced by other organisms such as bacteria, fungi and algae6. Forasmuch, many bioactive constituents in plants such as alkaloids, terpenoids, flavonoids, amino acids, enzymes, vitamins, proteins, and glycosides could also be a participant in bioreduction, formation and stabilization of the metal nanoparticles7,8,9.
Deverra tortuosa is a wild perennial bushy plant growing naturally in sandy and stony plains10. This plant is widespread in deserts of the Arabian ecoregion (Saudi Arabia, Palestine, Egypt, Libya and Tunisia). This plant Synonym is Pituranthos tortuosus (Desf.) and known in Arabic as Shabat El-Gabal or Qozzaah. As a member of Apiaceae family, D. tortuosa possesses a characteristic pungent or aromatic scent. The presence of important bioactive compounds in different parts of the plant; flavonoids, terpenoids, glycosides, essential oil, furanocoumarins and unsaturated sterols has been previously published11,12,13. The plant is often used by local citizens in traditional medicine as analgesic, carminative, diuretic, antiasthmatic and to relieve stomach pain and against intestinal parasites14,15. It has been found to be pharmacologically active to treat asthma, hepatitis, fever, rheumatism, diabetes, digestive difficulties and to regulate menstruation12,16. Also, it was reported to be used as an edible food to treat hypertension, against constipation and in the case of bites, and been used for grazing and fuel wood17,18. Another possible application could be its utilization in the nanobiotechnology field.
Among the metal nanoparticles ZnO.NPs are interesting due to its impressive properties from which the wide band gap, large binding energy and high piezoelectric property19. ZnO.NPs which can exhibit a wide variety of nanostructures are believed to be biosafe, nontoxic and biocompatiable, been used in various technologies and industries such as optoelectronics, piezoelectric and magnetic sensors, biodiagnosis, biological labelling, ceramic and rubber processing, environmental protection, biology and medicinal industry20,21,22. Chemically, the surface of ZnO is rich in -OH groups, which permit ZnO to slowly dissolve in both acidic (e.g., the tumor cells and tumor microenvironment) and strong basic conditions. Based on this property, ZnO.NPs have gained immense interest in biomedical23. The extended application of ZnO.NPs in medicine as anti-angiogenesis, antiplatelet agents, anti-inflammatory, dental materials, cosmetics, drug and gene delivery, have made ZnO.NPs a promising anticancer agent24,25.
Materials and Methods
General
All chemicals of high grade of purity were obtained from Sigma-Aldrich (St. Louis, MA, USA).All solutions were prepared with double distilled water. Analyses, qualitative and quantitative measurements involved the use of the following tools and systems: WARING COMMERCIAL Lab Blender (Dynamics Corp. of America, New Hartford, CT, USA), Hei-VAP Rotary evaporator (Heidolph, Germany),1260 Infinity II LC System (Agilent, USA),Ultrasonic homogenizer model 150/vt (Biologics Inc. Cary, North Carolina), UV-Visible Spectrophotometer (JASCO, Japan), FT/IR-4100 Spectrometer (JASCO, Japan), X’Pert PRO X-ray Diffraction System (PANalytical, Netherland), JEM-2100 High Resolution Transmission Electron Microscope (JEOL Ltd, Japan), Cellstar 96-well plate (Greiner Bio One International, Austria) and MR-96A microplate reader (Shenzhen Mindray Bio-Medical Electronics Co., Ltd, China).
Plant collection
The aerial parts of D. tortuosa were collected in July, 2017 from a natural ecosystem (30°20′47.8″N 31°37′51.1″E) at the Belbis City – 10th of Ramadan desert road, Sharqia governorate, Egypt and were identified by Prof. Dr. Hussien Abd El-Basset, Professor of Plant Taxonomy, Faculty of Science, Zagazig University and a voucher specimen was deposited in the herbarium of Botany Department, Zagazig University, Egypt.
Plant extraction
Plants were washed several times with double distilled water to remove any debris or particulates then shade dried at room temperature. Aerial parts were finely ground into a fine powder. The aqueous extract was prepared by the cold maceration method26. The plant powder (50 g) was soaked in 1 L of distilled water, kept in a shaker at 20 °C for 24 h for continuous agitation at 100 rpm for thorough mixing. Then the extract was filtered and stored at −4 °C for further investigations.
Phytochemical screening
Preliminary phytochemical screening of the extract was carried out to identify the active constituents, using standard methods27.
HPLC analysis
A high-performance liquid chromatography (HPLC) was used to detect, identify and quantify a number of phenolic compounds in the extract using an Agilent 1260 seriesfollowing a modified method by Wu et al.28. The separation was carried out using C18 column (4.6 mm ×250 mm i.d., 5 μm). The column temperature was maintained at 35 °C. The mobile phase consisted of water (A) and acetonitrile (B) at a flow rate 1 ml/min. The mobile phase was programmed consecutively in a linear gradient as follows: 0 min (80% A); 0–5 min (80% A); 5–8 min (40% A); 8–12 min (50% A); 12–14 min (80% A) and 14–16 min (80% A). The multi-wavelength detector was set at 280 nm. The injection volume was 10 μl for each of the sample solutions.
Green synthesis of zinc oxide nanoparticles
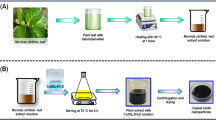
Biogenic synthesis of ZnO.NPs was carried out according to the method of Elumalai et al.29 with modifications. The crude plant extract (about 25 mL) was heated (60–80 °C) on a magnetic stirrer. When the temperature of the extract reached 60 °C, 2.5 g of zinc nitrate hexahydrate (Zn(NO3)2.6H2O) was added and left for about 1 h till a white precipitate appeared. This mixture then was left overnight in a hot air oven at 60 °C or till a creamy paste formed. This paste was collected and washed several times with a solution of distilled water: Ethanol (3:1). Afterwards, the collected paste was transferred to a ceramic crucible cup and heated in furnace at 400 °C for 2 h. The resultant white powder stored in an airtight container for characterization.
Characterization of ZnO.NPs
The ZnO.NPs initially analyzed by using the Rigol ultra-3660 UV-vis spectroscopy within the range 200–800 nm. Then FTIR was used to identify the functional groups and various phytochemical constituents involved in the reduction and stabilization of the synthesized nanoparticles. FTIR was carried out using the attenuated total reflectance (ATR) mode with a Jasco FTIR 4100 spectrophotometer (Japan). The results recorded in the range of 4000–400 cm−1. The powdered sample was subjected to a CuKα1-X Ray diffractometer radiation (λ = 1.5406 A°) operating at 40 kV and 30 mA with 2θ ranging from 30°–140° to confirm the presence of ZnO and analyze the crystallite structure and size. ZnO nanopowder was suspended in ethanol, sonicated then coated onto a copper grid and allowed to dry and examined by JEOL-2100 HR-TEM.
Cytotoxic activity
Cell lines
Human colorectal epithelial adenocarcinoma “Caco-2”, human lung epithelial carcinoma “A549”) and the normal human lung fibroblast cell line (WI38) were procured from tissue culture Lab in VACSERA Institute, Agoza, Egypt (The holding company for biological products and Vaccines).
Cell culture and MTT assay
To evaluate the cytotoxicity of the aqueous plant extract and ZnO.NPs, the MTT (3-(4,5- dimethylthiazol-2-yl– 2,5-diphenyl tetrazolium bromide) colorimetric assay was performed in 96-well plates30. The whole procedure was maintained under sterile conditions via the use of a laminar air-flow cabinet, following culturing and sub-culturing technique adopted by Thabrew et al.31.
Results
Phytochemical study
All results of phytochemical analysis are showed in Table 1. In the present study, the aqueous fraction from the crude extract showed positive results for triterpenes and/or steroids as measured by the Liebermann-Burchard reaction. It was found that D. tortuosa aqueous extract contained polyphenols and flavonoids, which may be responsible for the biological activities found.
HPLC analysis
HPLC analysis indicate the presence of Gallic acid, Chlorogenic acid, Caffeine, Coffeic acid, Syringic acid, Rutin, Ellagic acid, Coumaric acid, Vanillin, Ferulic acid, Naringenin, Propyl Gallate, Querectin and Cinnamic acid (Fig. 1& Table 2) that might have been responsible for their therapeutic potential. The amounts and structures of polyphenols are shown in Table 2 & Fig. 2.
Characterization of green synthesized ZnO.NPs
During synthesis, the change in color of the solution and formation of a yellowish-white precipitate was an indication that zinc nitrate had been reduced (Fig. S1).
U.V. spectrophotometric analysis
The formation of ZnO.NPs was initially confirmed by UV-vis spectroscopy within the range 200–800 nm. The absorption spectrum of green synthesized ZnO.NPs showed a characteristic peak at 374 nm (Fig. S2). The direct band gap (Eg) calculated was 3.32 eV.
FTIR spectroscopic analysis
FTIR is used as a confirmatory technique to the nanoparticle formation and offers an impression to the vibrational and rotational modes of the existing molecules, hence it helps to identify the functional and possible phytochemical molecules involved in the reduction and stabilization of ZnO.NPs. Fig. S3 representing the FTIR spectra of ZnO.NPs synthesized by the green approach showed a peak at 442 cm−1 which is corresponding to the hexagonal ZnO symmetric bending vibration and a peak at 878 cm−1 due to weak vibration of ZnO. The wide peaks present at 3434 cm−1 and 1117 cm−1 reflect the presence of OH and C—OH stretching vibrations respectively. Other smaller bond vibration peaks including 2925.48 cm−1 which denotes the C—H stretching vibration, 2352.73, 1630.52 and 1445.39 cm−1 are due to the presence of primary and secondary amines that are characteristics of proteins/enzymes and C–O stretching regions of polysaccharides and phenolic groups.
XRD analysis
X-ray diffraction pattern of synthesized ZnO.NPs was obtained as displayed in Fig. 3. The crystalline peaks positioned at (2θ) peaks angles of 31.80°, 34.45°, 36.28°, 47.59°, 56.65°, 62.94°, 66.46, 68.00°, 69.09, 72.57 and 77.0648° correspond to the reflection from (100), (002), (101), (102), (110), (103), (200), (112), (201), (004) to (202) crystal planes, respectively. By using Scherrer’s formula, the average crystallite size of ZnO which is derived from the FWHM of more intense peak corresponding to 101 plane located at 36.28° was estimated to be 15.41 nm.
HR-TEM analysis
TEM analysis can be used to understand the crystalline characteristics and size of the synthesized NPs. The analysis was carried out using JEOL-2100 and the images at different magnification (50 and 100 nm) are shown in Fig. 4. TEM images displaying the major series of particle size that were between 9.26 to 31.18 nm.
Cytotoxic assay
The cytotoxicity of the tested materials (D. tortuosa Aq. Ex. and ZnO.NPs) was investigated using Doxorubicin as a positive control, and the untreated cells were the negative control. From the MTT assay results present in Fig. 5, both the plant extract and ZnO.NPs showed a profound selective cytotoxic effect on the Caco-2 and A549 cancer cell lines with appreciable lower cytotoxic activity on the normal WI38 cells. This effect was concentration-dependent, where 1000 μg/ml had a larger effect than 500 μg/mL and so on. Besides, it was obvious that the extent of cytotoxicity influenced by the cell type and the material used. Markedly the ZnO.NPs had the most potent cytotoxic activity and Caco-2 was more sensitive than A549, as shown in Fig. 5. IC50 of A549 cells were 193.12 and 83.47 while, IC50 values of Caco-2 cells were 136.12 and 50.81 μg/mL by the extract and ZnO.NPs respectively. Significantly higher IC50 values 902.83 and 434.60 μg/ml obtained from the treatment of the normal lung epithelial cell (WI38) with the respective materials. While, nearly close IC50 values were observed among the different cell lines treated with Doxorubicin 145.26, 162.86, 186.10 μg/ml of Caco-2, A549 and WI38 cell lines, respectively. This is consistent with the microscopic examination data through which the cytotoxic activity could be distinguished by membrane blebbing, cell swelling or shrinkage, nuclear margination or fragmentation, and chromatin condensation (Figs. S4–S6).
Discussion
Plants considered an inimitable source for the future novel medicine and this value was found to be correlated with their active biocomponents32. In this regard, several phytoconstituents were identified in D. tortuosa aerial parts. Some important phenolic compounds were quantified with high prevalence of propyl Gallate and Naringenin. Thanks to their functional (hydroxyl) group, phenolics have been informed to be effective hydrogen donors which accounts for various biological activities33.
Based on the ability of plants to bioaccumulate metal ions and the involvement of active phytoconstituents as bioreductants and stabilizers, the green synthesis of ZnO.NPs could be illustrated34. The reduction of Zn nitrate into ZnO.NPs, which informed through the change in color, may be attributed to excitation of surface plasmon vibrations of nanoparticles which results in Surface Plasmon Resonance35.
According to previous studies suggested that ZnO.NPs exhibit a characteristic broad absorption peak between 330–460 nm36,37, peaking at 374 nm without any other peaks confirms the synthesis of pure ZnO.NPs with the aid of active biomolecules in the plant extract in reduction and stabilization of synthesized nanoparticles. This absorption peak could be attributed to the intrinsic band-gap absorption of ZnO due to the electron transitions from the valence band to the conduction band (O2p→Zn3d) as explained by Zak et al.38. Absorption in this wavelength of 374 nm further confirms that the absorption spectrum is slightly blue-shifted with respect to the bulk value (377 nm) of the ZnO NPs. This blue shift in the absorption edge is due to the quantum confinement effect among the individual nanoparticles. The good absorption of the ZnO-NPs in the UV region implies its applicability in the medical application such as sunscreen protectors or in antiseptic ointments39. The band gap and catalytic activity of metal oxide nanoparticles play a key role in their cytotoxic response to biological systems40,41.
From the FTIR results, appearance of other peaks may indicate the existence of enzymes, proteins and metabolites such as alkaloids, flavonoids, polyphenols and carboxylic acid “which remained bound to ZnO.NPs despite repeated washing”. These compounds, particularly flavonoids and other phenolics, assisted in the reduction of zinc ions to ZnO.NPs. The stability of the synthesized ZnO.NPs could presumably be accounted for the presence of free amino and carboxylic groups that have interacted with the zinc surface. Furthermore, the proteins present in the medium help in the stabilization of ZnO.NPs by forming a coat, covering the metal nanoparticles and preventing the nanoparticles agglomeration35,42 (Fig. S3).
All diffraction peaks of the sample obtained from XRD indicated the purity of ZnO nanocrystalline formation. In accordance with the JCPDS 36–1451card43, identical to the hexagonal phase with Wurtzite structures. The calculated particle size was found to lie within the size range of 9.26 to 31.18 nm obtained by TEM.
TEM images confirmed the hexagonal structure of the synthesized ZnO.NPs. This structure implies more ionicity and subsequent enhanced catalytic activity of the NP among the three 2D ZnO.NPs structures44. It is well documented that decreasing of particle size increases their functionality as antimicrobial and anticancer agent due to the larger surface-to-volume ratio45.
Cancer, the malignant case of uncontrolled cell proliferation, considered the second leading cause of death worldwide. Among the different cancer types; colorectal and lung cancers are alarming and being associated with the greatest mortality46. One of the most challenging fields in modern scientific research is developing new anticancer drugs with minimal side effects and enhanced selectivity and efficacy47. Ethno-medicine is as ancient as human civilization and many plant extracts and phytoconstituents have been identified to have antioxidant and anticancer properties. Earlier to the current results, Abdallah and Ezzat (2011), notified a similar cytotoxic activity of D. tortuosa essential oil against liver (HEPG2), colon HCT116), and breast cancer (MCF7). They related this activity to the high contents of terpinen-4-ol, γ-terpinene, sabinene, and β-myrcene13. Furthermore, phenols and polyphenolic compounds were found to be associated with the inhibition of cancer and atherosclerosis33,48. The phytoconstituents could exert their activity against carcinogens through the production of the Reactive Oxygen Species (ROS) which are involved in phagocytosis, regulation of cell proliferation and intracellular signaling49. On the other hand, many nano-medicine researches stated that ZnO nanostructures can be utilized to fight cancer cells, providing a possible target for the development of anti-tumor agents50,51 which support our observation that ZnO.NPs are dramatically less toxic to normal cells. Our finding that cytotoxicity depended on the cell type and the material used comes in full agreement with the previous studies13,52. Moreover, the present results confirmed the previously reported strong preferential cytotoxicity of ZnO.NPs to cancerous cells. A similar trend of selective cytotoxicity against three types of cancer cells, human hepatocellular carcinoma HepG2, human bronchial epithelial BEAS-2B and human lung adenocarcinoma A549 was reviewed by Akhtar et al.53. Likewise, ZnO.NPs exhibited a significant cytotoxic effect on HEp-2 cells as proclaimed by Jacob et al.54. Even though, the precise mechanism of cytotoxic activity of ZnO.NPs is yet under debate, several proposed ones are suggested and adopted. The basic mechanism behind the cytotoxicity of ZnO.NPs is the intracellular release of dissolved zinc ions, accompanied by ROS induction. This action is induced through a binary response: comprising the pro-inflammatory reaction of the cell against ZnO.NPs in addition to the characteristic surface property of the nanoparticle that makes ZnO.NPs act as a redox system24,55.
Conclusion
In this work, the aqueous extract of Deverra tortuosa was used for the green synthesis of ZnO nanoparticles. The phytochemical screening of D. tortuosa extract revealed copious kinds of active constituents with a high polyphenolic content that have the ability to chelate metal ions and aid in the bioreduction of ZnO.NPs. This promotes its utilization for the green synthesis of other metal NPs (e.g. Ag, Au). Moreover and to the best of our knowledge, still no published reports have investigated using D. tortuosa aqueous extract against the cancer cell lines (Caco-2 and A549). Different techniques were used for authenticating ZnO nanoparticles “UV–visible, FTIR, XRD and TEM” that confirmed the presence of nanoparticles with an average size of 15.22 nm. Both the aqueous extract of D. tortuosa and the synthesized ZnO nanoparticles showed an attractive selective cytotoxic activity against two tested cancer lines, offering satisfying ‘safer and cheaper’ alternatives to conventional therapy protocols. With all their promising characteristics of green synthesized ZnO.NPs, other biological activities should be evaluated. Moreover, the results of the current study need to be corroborated by testing these materials for their in vivo application.
References
Gunalana, S., Sivaraja, R. & Rajendranb, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci.: Mater. 22(6), 693–700 (2012).
Geonmonond, R. S., Da Silva, A. G. M. & Camargo, P. H. C. Controlled synthesis of noble metal nanomaterials: motivation, principles, and opportunities in nanocatalysis. An. Acad. Bras. Ciênc. 90, 719–744 (2018).
Sobha, D. K. et al. Emerging trends in nanobiotechnology. J. Biotech. Mol. Bio. Rev. 5(1), 1–12 (2010).
Xie, J., Lee, J. Y., Wang, D. I. C. & Ting, Y. P. Silver nanoplates: from biological to biomemetic synthesis. ACS Nano 1(5), 429–439 (2007).
Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 13(10), 2638–2650 (2011).
Ramesh, P., Rajendran, A. & Meenakshisundaram, M. Green Syntheis of Zinc Oxide Nanoparticles Using Flower Extract. Cassia Auriculata. J. Nanosci. Nanotechnol. 2(1), 41–45 (2014).
Iravani, S., Korbekandi, H. & Abbasi, S. Production of nanoparticles using organisms. Crit. Rev. Biotechnol. 29(4), 279–306 (2009).
Tolaymat, T. M. et al. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ. 408(5), 999–1006 (2010).
Makarov, V. V. et al. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat. 6(1), 35–44 (2014).
Boulos, L. Flora of Egypt. Vol. 2. AL-Hadara Publishing. Cairo, Egypt: 352 (2000).
Ahmed, Z. F., Wassel, G. M. & Abdel-Moneim, F. M. A preliminary phytochemical investigation of Pituranthos tortuosus (Desf.) Benth and Hook. J. Pharm. Sci. U.A.R. 10(1), 31–36 (1969).
Abdelwahed, A. et al. Chemical composition and antimicrobial activity of essential oils from Tunisian Pituranthos tortuosus (Coss.) Maire. Flavour Fragr. J. 21(1), 129–133 (2006).
Abdallah, H. M. & Ezzat, S. M. Effect of the method of preparation on the composition and cytotoxic activity of the essential oil of Pituranthos tortuosus. Z. Naturforsch C. 66(3-4), 143–148 (2011).
Boukef, K., Souissi, H. R. & Balansard, G. Contribution à l’étude des plantes utilisées en médicine traditionnelle tunisienne. Pl. Méd. Phyto. 16(4), 260–279 (1982).
Mahran, G. H., Ahmed, M. S., Seida, A. A. & Amarquaye, A. A. A phytochemical investigation of Pituranthos tortuosus (Desf.) Benth and Hook. Bull. Fac. Pharm. Cairo Uni. 27(1), 87–89 (1989).
Krifa, M., Gharad, T. & Haouala, R. Biological activities of essential oil, aqueous and organic extracts of Pituranthos tortuosus (Coss.) Maire. Sci. Hortic. 128(1), 61–67 (2011).
El-Mokasabi, F. M. Floristic composition and traditional uses of plant species at Wadi Alkuf, Al-Jabal Al-Akhder, Libya. American-Eurasian AEJAES 14(8), 685–697 (2014).
Bidak, L. M., Kamal, S. A., Halmy, M. W. A. & Heneidy, S. Z. Goods and services provided by native plants in desert ecosystems: examples from the north western coastal desert of Egypt. Glob. Ecol. Cons. 3, 433–447 (2015).
Vaseem, M. Umar, A. & Hahn, Y-B. Chapter 4 In Metal Oxide Nanostructures and Their Applications, edited by A. Umar and Y.-B. Hahn, American Scientific Publishers, New York, Vol. 5, pp. 1–36 (2010).
Theodore, L. Nanotechnology: Basic Calculations for Engineers and Scientists, Wiley, Hoboken (2006).
Wang, X., Lu, J., Xu, M. & Xing, B. Sorption of pyrene by regular and nanoscaled metal oxide particles: influence of adsorbed organic matter. Environ. Sci. Technol. 42(19), 7267–7272 (2008).
Dagdeviren, C. et al. Transient, biocompatible electronics and energy harvesters based on ZnO. Small 9(20), 3398–3404 (2013).
Liu, D. et al. Surface Functionalization of ZnO Nanotetrapods with Photoactive and Electroactive Organic Monolayers. Langmuir 24(9), 5052–5059 (2008).
Bisht, G. & Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine (Rij) 3, 9–20 (2016).
Sonia, S., Linda Jeeva Kumari, H., Ruckmanib, K. & Sivakumara, M. Antimicrobial and antioxidant potentials of biosynthesized colloidal zinc oxide nanoparticles for a fortified cold cream formulation: A potent nanocosmeceutical application. Mater. Sci. Eng. C Mater. Biol. Appl. 79, 581–589 (2017).
Azwanida, N. N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 4(3), 196–201 (2015).
Iqbal, E., Abu Salim, K. & Lim, L. B. L. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud. Univ.–Sci. 27(3), 224–232 (2015).
Wu, S. et al. Carthamus red from Carthamus tinctorius L. exerts antioxidant and hepatoprotective effect against ccl4-induced liver damage in rats via the Nrf2 pathway. J. Ethnopharmacol. 148(9), 570–578 (2013).
Elumalai, K., Velmurugan, S., Ravi, S., Kathiravan, V. & Ashokkumar, S. Green synthesis of zinc oxide nanoparticles using Moringa oleifera leaf extract and evaluation of its antimicrobial activity. Spectrochimica Acta Part A: Mol. Biomol. Spectro. 143, 158–164 (2015).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65(1-2), 55–63 (1983).
Thabrew, M. I., Mitry, R. R., Morsy, M. A. & Hughes, R. D. Cytotoxic effects of a decoction of Nigella sativa, Hemidesmus indicus, and Smilax glabra on human hepatoma Hep G2 cells. Life Sci. 77(12), 1319–1330 (2005).
Dar, R. A., Shahnawaz, M. & Qazi, P. H. General overview of medicinal plants: A review. J. Phytopharmacol. 6(6), 349–351 (2017).
Pradhan, D. Pharmacological effect of some fractions obtained from Sapindus trifoliatus acting as an antioxidant and against mammary cell proliferation. Afr. J. Pharm. Pharmacol. 8(17), 455–463 (2014).
Hassan, S. S. M., El Azab, W. I. M., Ali, H. R. & Mansour, M. S. M. Green synthesis and characterization of ZnO nanoparticles for photocatalytic degradation of anthracene. Adv. Nat. Sci: Nanosci. Nanotechnol. 6(4), 045012 (11pp) (2015).
Datta, A. et al. Green synthesis of Zinc Oxide nanoparticles using Parthenium hysterophorus leaf extract and evaluation of their antibacterial properties. J. Biotechnol. Biomater. 7(3), 271–276 (2017).
Nagarajan, S. & Kuppusamy, K. A. Extracellular synthesis of zinc oxide nanoparticle using seaweeds of Gulf of Mannar, India. J. Nanobiotechnol. 11(1), 11–39 (2013).
Dobrucka, R., Dlugaszewska, J. & Kaczmarek, M. Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using of Chelidonium majus extract. Biomed. Microdevices 20(1), 5–18 (2018).
Zak, A. K., Abd.Majid, W. H., Mahmoudian, M. R., Darroudi, M. & Yousefi, R. Starch-stabilized synthesis of ZnO nanopowders at low temperature and optical properties study. Adv. Powder Technol. 24(3), 618–624 (2013).
Jiang, J., Pi, J. & Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. vol. 2018, Article ID 1062562, 18 pages https://doi.org/10.1155/2018/1062562 (2018).
Zhang, H. et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 6(5), 4349–4368 (2012).
Punnoose, A. et al. Cytotoxicity of ZnO nanoparticles can be tailored by modifying their surface structure: A green chemistry approach for safer nanomaterials. ACS Sustain. Chem. Eng. 2(7), 1666–1673 (2014).
Sindhura, K. S., Prasad, T. N. V. K. V., Selvam, P. P. & Hussain, O. M. Synthesis, characterization and evaluation of effect of phytogenic zinc nanoparticles on soil exo-enzymes. Appl. Nanosci. 4(7), 819–827 (2014).
American Society for Testing and Material, Powder Diffraction Files, Joint Committee on Powder Diffraction Standards, Swarthmore, PA, 3 (1999).
Supatutkul, C., Pramchu, S., Jaroenjittichai, A. P. & Laosiritaworn, Y. Electronic properties of two-dimensional zinc oxide in hexagonal, (4, 4)-tetragonal, and (4, 8)-tetragonal structures by using Hybrid Functional calculation. J. Phys.; Conf. Ser. 901(1), 012172, https://doi.org/10.1088/1742-6596/901/1/012172 (2017).
Azizi, S., Mohamad, R. & Shahri, M. M. Green microwave-assisted combustion synthesis of Zinc Oxide nanoparticles with Citrullus colocynthis (L.) Schrad: Characterization and Biomedical Applications. Molecules 22(2), 301–314 (2017).
WHO. World Health Organization. Geneva, Switzerland, – The International Agency for Research on Cancer (IARC) 12 September 2018. http://gco.iarc.fr/tomorrow/home (2018).
Ali, R. et al. New anticancer agents: Recent developments in tumor therapy. Anticancer Res. 32(7), 2999–3005 (2012).
Rao, G. V., Kumar, S., Islam, M. & Mansour, S. E. Folk medicines for anticancer therapy- acurrent status. J. Cancer Ther. 6(2), 913–922 (2008).
Ibrahim, A. Y., Mahmoud, K. & El-Hallouty, S. M. Screening of antioxidant and cytotoxicity activities of some plant extracts from Egyptian flora. J. Appl. Sci. Res. 7(7), 1246–1258 (2011).
Wahab, R. et al. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B Biointerfaces 117, 267–276 (2014).
Moghaddam, A. B. et al. Eco-Friendly Formulated Zinc Oxide Nanoparticles: Induction of Cell Cycle Arrest and Apoptosis in the MCF-7 Cancer Cell Line. Genes 8(10), 281–296 (2017).
Krifa, M. et al. Immunomodulatory and anticancer effects of Pituranthos tortuosus essential oil. Tumor. Biol. 36(7), 5165–5170 (2015).
Akhtar, M. J. et al. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomedicine 7, 845–857 (2012).
Jacob, S. J. P., Bharathkumar, R. & Ashwathram, G. Aspergillus niger mediated synthesis of ZnO Nanoparticles and their antimicrobial and in vitro Anticancerous activity. WJPR 3(2), 3044–3054 (2014).
Rasmussen, J. W., Martinez, E., Louka, P. & Wingett, D. G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug. Deliv. 7(9), 1063–1077 (2010).
Acknowledgements
We thank staff of National Research Centre, El-Tahrir St., Dokki, Cairo 12622, Egypt for conducting the analysis and determining the results of nanoparticles. Guest or honorary authorship based solely on position (e.g. research supervisor, departmental head) is discouraged.
Author information
Authors and Affiliations
Contributions
Y.S. and M.Aa. Conceived and designed the experiments; Y.S. and M.M. performed the experiments; M.Az. analyzed the data; Y.S. and M.Aa. Contributed reagents/materials/analysis tools; Y.S., M.Aa., I.R. and M.Az. wrote the paper. Y.S. and I.R. reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Selim, Y.A., Azb, M.A., Ragab, I. et al. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra tortuosa and their Cytotoxic Activities. Sci Rep 10, 3445 (2020). https://doi.org/10.1038/s41598-020-60541-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60541-1
This article is cited by
-
Comprehensive antifungal investigation of green synthesized silver nanoformulation against four agriculturally significant fungi and its cytotoxic applications
Scientific Reports (2024)
-
Biogenic synthesis of ZnO nanoparticles using Polystichum squarrosum extract and its applications as anti-oxidant, anti-diabetic agent and industrial waste water treatment
Emergent Materials (2024)
-
Biomass nanoarchitectonics using an agro waste extract for biological performance of samarium doped zinc oxide nanoparticles
Applied Physics A (2024)
-
Zinc Nanoparticles for Enhancing Plant Tolerance to Abiotic Stress: A Bibliometric Analysis and Review
Journal of Soil Science and Plant Nutrition (2024)
-
Characterization and nematicidal potential of copper, iron and zinc nanoparticles synthesized from Tridax procumbens L. Extract on Meloidogyne incognita infected cabbage plants
European Journal of Plant Pathology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.