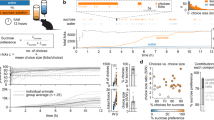
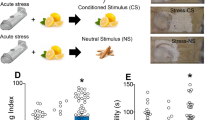
Abstract
Anhedonia is the inability to experience pleasure from rewarding or enjoyable activities and is a core symptom of depression in humans. Here, we describe a protocol for the measurement of anhedonia in mice, in which anhedonia is measured by a sucrose preference test (SPT) based on a two-bottle choice paradigm. A reduction in the sucrose preference ratio in experimental relative to control mice is indicative of anhedonia. To date, inconsistent and variable results have been reported following the use of the SPT by different groups, probably due to the use of different protocols and equipment. In this protocol, we describe how to set up a clearly defined apparatus for SPT and provide a detailed protocol to ensure greater consistency when carrying out SPT. This optimized protocol is highly sensitive, reliable, and adaptable for evaluation of chronic stress–related anhedonia, as well as morphine-induced dependence. The whole SPT, including adaptation, baseline measurement, and testing, takes 8 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Krishnan, V. & Nestler, E. J. The molecular neurobiology of depression. Nature 455, 894–902 (2008).
Pariante, C. M. & Lightman, S. L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 31, 464–468 (2008).
Seong, E., Seasholtz, A. F. & Burmeister, M. Mouse models for psychiatric disorders. Trends Genet. 18, 643–650 (2002).
Bourin, M., Fiocco, A. J. & Clenet, F. How valuable are animal models in defining antidepressant activity? Hum. Psychopharmacol. 16, 9–21 (2001).
Tanti, A. & Belzung, C. Open questions in current models of antidepressant action. Br. J. Pharmacol. 159, 1187–1200 (2010).
Nestler, E. J. et al. Preclinical models: status of basic research in depression. Biol. Psychiatry 52, 503–528 (2002).
Griebel, G. et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc. Natl Acad. Sci. USA 99, 6370–6375 (2002).
Cryan, J. F., Mombereau, C. & Vassout, A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 29, 571–625 (2005).
Porsolt, R. D., Le Pichon, M. & Jalfre, M. Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732 (1977).
Hasegawa, H. & Tomita, H. Assessment of taste disorders in rats by simultaneous study of the two-bottle preference test and abnormal ingestive behavior. Auris, Nasus, Larynx 13, S33–S41 (1986).
Goshen, I. et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 13, 717–728 (2008).
Zhou, Q. G. et al. Hippocampal telomerase is involved in the modulation of depressive behaviors. J. Neurosci. 31, 12258–12269 (2011).
Sobrian, S. K., Marr, L. & Ressman, K. Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 501–518 (2003).
Huang, H. J. et al. Ghrelin alleviates anxiety- and depression-like behaviors induced by chronic unpredictable mild stress in rodents. Behav. Brain Res. 326, 33–43 (2017).
Liu, X. L. et al. Fluoxetine regulates mTOR signalling in a region-dependent manner in depression-like mice. Sci. Rep. 5, 16024 (2015).
Tordoff, M. G. & Rabusa, S. H. Calcium-deprived rats avoid sweet compounds. J. Nutr. 128, 1232–1238 (1998).
Bertino, M. & Wehmer, F. Dietary influences on the development of sucrose acceptability in rats. Dev. Psychobiol. 14, 19–28 (1981).
Tordoff, M. G. & Bachmanov, A. A. Influence of test duration on the sensitivity of the two-bottle choice test. Chem. Senses 27, 759–768 (2002).
Eagle, A. L., Mazei-Robison & Robison, A. J. Sucrose preference test to measureStress - induced anhedonia. Bio-protocol 6, 1–6 (2016).
Koo, J. W., Russo, S. J., Ferguson, D., Nestler, E. J. & Duman, R. S. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl Acad. Sci. USA 107, 2669–2674 (2010).
Zhou, Q. G. et al. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J. Neurochem. 103, 1843–1854 (2007).
Zhou, Q. G. et al. Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. J. Neurosci. 31, 7579–7590 (2011).
Zhou, Q. G. et al. Hippocampal telomerase is involved in the modulation of depressive behaviors. J. Neurosci. 31, 12258–12269 (2011).
Zhou, Q. G. et al. Reactivation of Tert in the medial prefrontal cortex and hippocampus rescues aggression and depression of Tert(-/-) mice. Transl. Psychiatry 6, e836 (2016).
Cryan, J. F., Markou, A. & Lucki, I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 23, 238–245 (2002).
Zhou, Q. & Liu, M. Doubt about antidepressant-like effect. J. Biomed. Res. 27, 245–248 (2013).
Zhang, J. et al. Neuronal nitric oxide synthase alteration accounts for the role of 5-HT1A receptor in modulating anxiety-related behaviors. J. Neurosci. 30, 2433–2441 (2010).
Santarelli, L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 (2003).
Forbes, N. F., Stewart, C. A., Matthews, K. & Reid, I. C. Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol. Behav. 60, 1481–1484 (1996).
Berton, O. et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 (2006).
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007).
Mourlon, V. et al. Maternal deprivation induces depressive-like behaviours only in female rats. Behav. Brain Res. 213, 278–287 (2010).
Ducottet, C., Griebel, G. & Belzung, C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 625–631 (2003).
Zhou, Q. G. et al. Reactivation of Tert in the medial prefrontal cortex and hippocampus rescues aggression and depression of Tert(-/-) mice. Transl. Psychiatry 6, e836 (2016).
Hurst, J. L. & West, R. S. Taming anxiety in laboratory mice. Nat. Methods 7, 825–826 (2010).
Malatynska, E. et al. Anhedonic-like traits and lack of affective deficits in 18-month-old C57BL/6 mice: implications for modeling elderly depression. Exp. Gerontol. 47, 552–564 (2012).
Mateus-Pinheiro, A. et al. The sweet drive test: refining phenotypic characterization of anhedonic behavior in rodents. Front. Behav. Neurosci. 8, 74 (2014).
Ducottet, C., Griebel, G. & Belzung, C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 625–631 (2003).
Bohn, L. M., Gainetdinov, R. R., Lin, F. T., Lefkowitz, R. J. & Caron, M. G. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408, 720–723 (2000).
Cui, Y. et al. Targeted expression of μ-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat. Neurosci. 17, 254–261 (2014).
Devilliers, M. et al. Activation of TREK-1 by morphine results in analgesia without adverse side effects. Nat. Commun. 4, 2941 (2013).
Stromberg, M. F., Meister, S., Volpicelli, J. R. & Ulm, R. R. Morphine enhances selection of both sucrose and ethanol in a two-bottle test. Alcohol 14, 55–62 (1997).
Czirr, S. A. & Reid, L. D. Demonstrating morphine's potentiating effects on sucrose-intake. Brain Res. Bull. 17, 639–642 (1986).
Valverde, O. et al. Modulation of anxiety-like behavior and morphine dependence in CREB-deficient mice. Neuropsychopharmacology 29, 1122–1133 (2004).
Gallego, X. et al. Increased opioid dependence in a mouse model of panic disorder. Front. Behav. Neurosci. 3, 60 (2010).
Acknowledgements
This research was supported by grants from the National Key Research and Development Program of China (2016YFC1306703 to D.-Y.Z.), the National Natural Science Foundation of China (81571269 to Q.-G.Z., 31530091 to D.-Y.Z., 31771334 to H.C., and 31671107 to L.-J.Z.), the Major Research Plan of the National Natural Science Foundation of China (91649125 to H.C.), and the Natural Science Foundation of Jiangsu Province (BK20170021 to L.-J.Z.). This behavioral procedure was adapted from previously published studies7 and was improved and performed by our group as described12,22,34. This study was also supported by the Key Lab of Cardiovascular and Cerebrovascular Drugs of Jiangsu Province and by the Collaborative Innovation Center For Cardiovascular Disease Translational Medicine for data collection, analysis, and interpretation.
Author information
Authors and Affiliations
Contributions
M.-Y.L., C.-Y.Y., L.-J.Z., C.X. and X.-H.Z. acquired the data. C.-X.L. gave advice for revision of the article. D.-Y.Z. and H.C. provided materials for the experiments. Q.-G.Z., D.-Y.Z., and H.C. prepared and wrote the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol:
Zhou, Q. G. et al. J. Neurosci. 31, 7579–7590 (2011): https://doi.org/10.1523/JNEUROSCI.0004-11.2011
Zhou, Q. G. et al. J. Neurosci. 31, 12258–12269 (2011): https://doi.org/10.1523/JNEUROSCI.0805-11.2011
Zhou, Q. G. et al. Transl. Psychiatry. 6, e836 (2016): https://doi.org/10.1038/tp.2016.106
Integrated supplementary information
Supplementary Figure 1 The weight of mice is altered by CMS and fluoxetine.
(a) The weight of mice exposed to 28-day CMS is lower than those of control mice. n = 15. (b) Exposure to 56-day CMS led to decreased weight of mice, reversed by fluoxetine treatment. n = 15. *P < 0.01, **P < 0.01, ***P < 0.001. Student’s t test for a. One-way ANOVA for b. Error bars, s.e.m. All the experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University
Supplementary Figure 2 Associated intakes of sucrose and regular water solutions altered by CMS and fluoxetine.
(a) The intakes of sucrose and regular water solutions in SPT before CMS. n = 11 – 12 for day 1, n = 12 – 14 for day 2, and n = 14 – 15 in each group for test. (b) The intakes of sucrose and regular water solutions in SPT after 28 days CMS exposure. n = 12-14 for day 1, n = 14 for day 2, and n = 15 in each group for test. (c) The intakes of sucrose and regular water solutions in SPT after 56 days CMS exposure with or without fluoxetine treatment during day 22 – 56. n = 12-13 for day 1, n = 13 – 14 for day 2, and n = 13 – 15 in each group for test. Invalid data caused by several conditions including no drinking, over drinking, and no preference was excluded in according to the criteria described in the protocol. Error bars, s.e.m. All the experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University
Supplementary Figure 3 Associated intakes of sucrose and regular water solutions altered by CSDS.
(a) The intakes of sucrose and regular water solutions in SPT after CSDS. n = 12 – 13 for day 1, n = 13 – 14 for day 2, n = 14 for test. Invalid data caused by several conditions including no drinking, over drinking, and no preference was excluded in according to the criteria described in the protocol. Error bars, s.e.m. All the experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University
Supplementary Figure 4 Associated intakes of sucrose and regular water solutions altered by morphine.
(a) The intakes of sucrose and regular water solutions in SPT after morphine dependence. n = 11 – 12 for day 1, n = 12 – 14 for day 2, n = 15 for test. (b) The intakes of sucrose and regular water solutions in SPT after morphine withdrawal. n = 12 – 14 for day 1, n = 15 for day 2, n = 15 for test. Error bars, s.e.m. Invalid data caused by several conditions including no drinking, over drinking, and no preference was excluded in according to the criteria described in the protocol. All the experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University
Supplementary Figure 5 Global withdrawal score after morphine withdrawal.
(a) The global withdrawal score of mice treated with saline or morphine. Withdrawal was precipitated in both groups by the administration of the opioid receptor antagonist, naloxone (1 mg/kg, s.c.). Somatic signs of withdrawal, including jumping, wet dog shakes, paw tremor, teeth chattering, body tremor, ptosis, piloerection, and sniffing, were recorded for 30 minutes immediately after administration. The global withdrawal score was calculated for each animal by giving a relative weight to each withdrawal sign. n = 15 in each group. ***P < 0.001. Student’s t test. Error bars, s.e.m. All the experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–5, Supplementary Methods, and Supplementary Table 1
Rights and permissions
About this article
Cite this article
Liu, MY., Yin, CY., Zhu, LJ. et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 13, 1686–1698 (2018). https://doi.org/10.1038/s41596-018-0011-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0011-z
This article is cited by
-
Neuroprotective effect of bromelain on BDNF-TRKB signalling pathway in chronic unpredictable stress-induced depression model
Beni-Suef University Journal of Basic and Applied Sciences (2024)
-
Antidepressant-like effects of psychedelics in a chronic despair mouse model: is the 5-HT2A receptor the unique player?
Neuropsychopharmacology (2024)
-
Prolonged HPA axis dysregulation in postpartum depression associated with adverse early life experiences: a cross-species translational study
Nature Mental Health (2024)
-
Characterization of graded 6-Hydroxydopamine unilateral lesion in medial forebrain bundle of mice
Scientific Reports (2024)
-
Nutritional ketosis as treatment for alcohol withdrawal symptoms in female C57BL/6J mice
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.