Key Points
-
Nephritis remains a major cause of morbidity and mortality in systemic lupus erythematosus (SLE), with both extrinsic and intrinsic renal factors influencing long-term prognosis
-
Renal injury involves local immune responses and aberrant responses by the renal parenchyma; some of these responses are not reversed by immunosuppressive therapy and might require targeting of nonimmune mechanisms
-
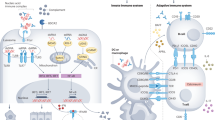
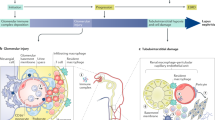
Although lupus nephritis is initiated by immune complex deposition, the response to injury in individuals varies owing to differences in immune mechanisms, inflammation versus metabolic changes, hypoxia, and tissue repair and fibrosis
-
Disease phenotyping based on mechanism of renal injury, which are being discovered by genetic analyses, molecular profiling and proteomic analyses, could suggest new and more individualized therapeutic approaches
-
Studies in mouse models show that progression to chronic kidney disease is not inevitable even if systemic autoimmunity persists, and that several checkpoints in the progression pathway are amenable to therapy
-
Because clinical remission and renal histologic remission are not always correlated, improved disease monitoring and/or repeated biopsies could help identify patients at risk of progression and in need of high-intensity maintenance therapy
Abstract
Despite marked improvements in the survival of patients with severe lupus nephritis over the past 50 years, the rate of complete clinical remission after immune suppression therapy is <50% and renal impairment still occurs in 40% of affected patients. An appreciation of the factors that lead to the development of chronic kidney disease following acute or subacute renal injury in patients with systemic lupus erythematosus is beginning to emerge. Processes that contribute to end-stage renal injury include continuing inflammation, activation of intrinsic renal cells, cell stress and hypoxia, metabolic abnormalities, aberrant tissue repair and tissue fibrosis. A deeper understanding of these processes is leading to the development of novel or adjunctive therapies that could protect the kidney from the secondary non-immune consequences of acute injury. Approaches based on a molecular–proteomic–lipidomic classification of disease should yield new information about the functional basis of disease heterogeneity so that the most effective and least toxic treatment regimens can be formulated for individual patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ortega, L. M., Schultz, D. R., Lenz, O., Pardo, V. & Contreras, G. N. Review: lupus nephritis: pathologic features, epidemiology and a guide to therapeutic decisions. Lupus 19, 557–574 (2010).
Markowitz, G. S. & D'Agati, V. D. Classification of lupus nephritis. Curr. Opin. Nephrol. Hypertens. 18, 220–225 (2009).
Weening, J. J. et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 65, 521–530 (2004).
Schwartz, M. M. et al. Irreproducibility of the activity and chronicity indices limits their utility in the management of lupus nephritis. Am. J. Kidney Dis. 21, 374–377 (1993).
Austin, H. A. 3rd, Muenz, L. R., Joyce, K. M., Antonovych, T. T. & Balow, J. E. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 25, 689–695 (1984).
Rovin, B. H., Parikh, S. V. & Alvarado, A. The kidney biopsy in lupus nephritis: is it still relevant? Rheum. Dis. Clin. North Am. 40, 537–552 (2014).
Vandepapeliere, J. et al. Prognosis of proliferative lupus nephritis subsets in the Louvain Lupus Nephritis inception Cohort. Lupus 23, 159–165 (2014).
Alvarado, A. et al. The value of repeat kidney biopsy in quiescent Argentinian lupus nephritis patients. Lupus 23, 840–847 (2014).
Zickert, A., Sundelin, B., Svenungsson, E. & Gunnarsson, I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci. Med. 1, e000018 (2014).
Dubois, E. L. in Lupus Erythematosus (ed. Dubois, E. L.) 72–89 (USC Press, 1974).
Contreras, G. et al. Factors associated with poor outcomes in patients with lupus nephritis. Lupus 14, 890–895 (2005).
Dooley, M. A. et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N. Engl. J. Med. 365, 1886–1895 (2011).
Ginzler, E. M. et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res. Ther. 14, R33 (2012).
Houssiau, F. A. & Lauwerys, B. R. Current management of lupus nephritis. Best Pract. Res. Clin. Rheumatol. 27, 319–328 (2013).
Schwartz, M. M. The pathology of lupus nephritis. Semin. Nephrol. 27, 22–34 (2007).
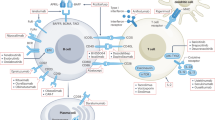
Madaio, M. P. The role of autoantibodies in the pathogenesis of lupus nephritis. Semin. Nephrol. 19, 48–56 (1999).
Kalaaji, M., Sturfelt, G., Mjelle, J. E., Nossent, H. & Rekvig, O. P. Critical comparative analyses of anti-α-actinin and glomerulus-bound antibodies in human and murine lupus nephritis. Arthritis Rheum. 54, 914–926 (2006).
Hedberg, A., Mortensen, E. S. & Rekvig, O. P. Chromatin as a target antigen in human and murine lupus nephritis. Arthritis Res. Ther. 13, 214 (2011).
Trouw, L. A. et al. Anti-C1q autoantibodies deposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexes. J. Clin. Invest. 114, 679–688 (2004).
Tsirogianni, A., Pipi, E. & Soufleros, K. Relevance of anti-C1q autoantibodies to lupus nephritis. Ann. NY Acad. Sci. 1173, 243–251 (2009).
Ullal, A. J. et al. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J. Autoimmun. 36, 173–180 (2011).
Hakkim, A. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA 107, 9813–9818 (2010).
Seredkina, N. & Rekvig, O. P. Acquired loss of renal nuclease activity is restricted to DNaseI and is an organ-selective feature in murine lupus nephritis. Am. J. Pathol. 179, 1120–1128 (2011).
Vlahakos, D. V. et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 41, 1690–1700 (1992).
Liang, Z. et al. Pathogenic profiles and molecular signatures of antinuclear autoantibodies rescued from NZM2410 lupus mice. J. Exp. Med. 199, 381–398 (2004).
Moroni, G. et al. The value of a panel of autoantibodies for predicting the activity of lupus nephritis at time of renal biopsy. J. Immunol. Res. 2015, 106904 (2015).
Bergtold, A., Gavhane, A., D'Agati, V., Madaio, M. & Clynes, R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J. Immunol. 177, 7287–7295 (2006).
Perez de Lema, G. et al. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J. Am. Soc. Nephrol. 12, 1369–1382 (2001).
Segerer, S. & Schlondorff, D. Role of chemokines for the localization of leukocyte subsets in the kidney. Semin. Nephrol. 27, 260–274 (2007).
Barrat, F. J. et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202, 1131–1139 (2005).
Teichmann, L. L., Schenten, D., Medzhitov, R., Kashgarian, M. & Shlomchik, M. J. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity 38, 528–540 (2013).
Bethunaickan, R. et al. Identification of stage-specific genes associated with lupus nephritis and response to remission induction in (NZB × NZW)F1 and NZM2410 mice. Arthritis Rheumatol. 66, 2246–2258 (2014).
Tesch, G. H., Maifert, S., Schwarting, A., Rollins, B. J. & Kelley, V. R. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Faslpr mice. J. Exp. Med. 190, 1813–1824 (1999).
Bethunaickan, R. et al. Anti-TNF treatment of IFN induced lupus nephritis reduces the renal macrophage response but does not alter glomerular immune complex formation. Arthritis Rheum. 64, 3399–3408 (2012).
Liu, J. et al. Genomic view of systemic autoimmunity in MRLlpr mice. Genes Immun. 7, 156–168 (2006).
Berthier, C. C. et al. Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. J. Immunol. 189, 988–1001 (2012).
Migliorini, A. et al. The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am. J. Pathol. 183, 431–440 (2013).
Gurkan, S. et al. Inhibition of type I interferon signalling prevents TLR ligand-mediated proteinuria. J. Pathol. 231, 248–256 (2013).
Boswell, J. M., Yui, M. A., Burt, D. W. & Kelley, V. E. Increased tumor necrosis factor and IL-1 β gene expression in the kidneys of mice with lupus nephritis. J. Immunol. 141, 3050–3054 (1988).
Herrera-Esparza, R., Barbosa-Cisneros, O., Villalobos-Hurtado, R. & Avalos-Diaz, E. Renal expression of IL-6 and TNFα genes in lupus nephritis. Lupus 7, 154–158 (1998).
Zhao, J. et al. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 65, 3176–3185 (2013).
Kahlenberg, J. M. & Kaplan, M. J. The inflammasome and lupus: another innate immune mechanism contributing to disease pathogenesis? Curr. Opin. Rheumatol. 26, 475–481 (2014).
Zhao, J. et al. Lupus nephritis: glycogen synthase kinase 3β promotion of renal damage through activation of the NLRP3 inflammasome in lupus-prone mice. Arthritis Rheumatol. 67, 1036–1044 (2015).
Reiser, J. et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J. Clin. Invest. 113, 1390–1397 (2004).
Amarilyo, G., Lourenco, E. V., Shi, F. D. & La Cava, A. IL-17 promotes murine lupus. J. Immunol. 193, 540–543 (2014).
Schmidt, T. et al. Function of the TH17/interleukin-17A immune response in murine lupus nephritis. Arthritis Rheumatol. 67, 475–487 (2015).
Chang, A. et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J. Immunol. 186, 1849–1860 (2011).
Kinloch, A. J. et al. Vimentin is a dominant target of in situ humoral immunity in human lupus tubulointerstitial nephritis. Arthritis Rheumatol. 66, 3359–3370 (2014).
Winchester, R. et al. Immunologic characteristics of intrarenal T cells: trafficking of expanded CD8+ T cell β-chain clonotypes in progressive lupus nephritis. Arthritis Rheum. 64, 1589–1600 (2012).
Tucci, M., Stucci, S., Strippoli, S. & Silvestris, F. Cytokine overproduction, T-cell activation, and defective T-regulatory functions promote nephritis in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 457146 (2010).
Enghard, P. et al. Urinary CD4 T cells identify SLE patients with proliferative lupus nephritis and can be used to monitor treatment response. Ann. Rheum. Dis. 73, 277–283 (2014).
Rose, M. L. Role of anti-vimentin antibodies in allograft rejection. Hum. Immunol. 74, 1459–1462 (2013).
Bruschi, M. et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo: α-enolase and annexin AI. J. Am. Soc. Nephrol. 25, 2483–2498 (2014).
Lech, M. & Anders, H. J. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta 1832, 989–997 (2013).
Duffield, J. S. Macrophages in kidney repair and regeneration. J. Am. Soc. Nephrol. 22, 199–201 (2011).
Hill, G. S. et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 59, 304–316 (2001).
Bethunaickan, R. et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J. Immunol. 186, 4994–5003 (2011).
Anders, H. J. et al. Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J. Am. Soc. Nephrol. 15, 1504–1513 (2004).
Lin, S. L., Castano, A. P., Nowlin, B. T., Lupher, M. L. Jr & Duffield, J. S. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J. Immunol. 183, 6733–6743 (2009).
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Sahu, R., Bethunaickan, R., Singh, S. & Davidson, A. Structure and function of renal macrophages and dendritic cells from lupus-prone mice. Arthritis Rheumatol. 66, 1596–1607 (2014).
Heymann, F. et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J. Clin. Invest. 119, 1286–1297 (2009).
Kassianos, A. J. et al. Increased tubulointerstitial recruitment of human CD141hi CLEC9A+ and CD1c+ myeloid dendritic cell subsets in renal fibrosis and chronic kidney disease. Am. J. Physiol. Renal Physiol. 305, F1391–F1401 (2013).
Salmon, A. H., Neal, C. R. & Harper, S. J. New aspects of glomerular filtration barrier structure and function: five layers (at least) not three. Curr. Opin. Nephrol. Hypertens. 18, 197–205 (2009).
Fu, J., Lee, K., Chuang, P. Y., Liu, Z. & He, J. C. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am. J. Physiol. Renal Physiol. 308, F287–F297 (2015).
Khan, S. et al. Mesangial cell integrin αvβ8 provides glomerular endothelial cell cytoprotection by sequestering TGF-β and regulating PECAM-1. Am. J. Pathol. 178, 609–620 (2011).
Floege, J., Eitner, F. & Alpers, C. E. A new look at platelet-derived growth factor in renal disease. J. Am. Soc. Nephrol. 19, 12–23 (2008).
Daehn, I. et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J. Clin. Invest. 124, 1608–1621 (2014).
Kriz, W. & LeHir, M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 67, 404–419 (2005).
Kida, Y., Ieronimakis, N., Schrimpf, C., Reyes, M. & Duffield, J. S. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J. Am. Soc. Nephrol. 24, 559–572 (2013).
Alon, R. & Nourshargh, S. Learning in motion: pericytes instruct migrating innate leukocytes. Nat. Immunol. 14, 14–15 (2013).
Kramann, R. & Humphreys, B. D. Kidney pericytes: roles in regeneration and fibrosis. Semin. Nephrol. 34, 374–383 (2014).
Schrimpf, C., Teebken, O. E., Wilhelmi, M. & Duffield, J. S. The role of pericyte detachment in vascular rarefaction. J. Vasc. Res. 51, 247–258 (2014).
Padberg, J. S. et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 234, 335–343 (2014).
Kuwabara, A., Satoh, M., Tomita, N., Sasaki, T. & Kashihara, N. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia 53, 2056–2065 (2010).
Dimke, H. et al. Tubulovascular cross-talk by vascular endothelial growth factor A maintains peritubular microvasculature in kidney. J. Am. Soc. Nephrol. 26, 1027–1038 (2014).
Kumpers, P. et al. The Tie2 receptor antagonist angiopoietin 2 facilitates vascular inflammation in systemic lupus erythematosus. Ann. Rheum. Dis. 68, 1638–1643 (2009).
Kida, Y., Tchao, B. N. & Yamaguchi, I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr. Nephrol. 29, 333–342 (2014).
Gilkeson, G. S. et al. Endothelial nitric oxide synthase reduces crescentic and necrotic glomerular lesions, reactive oxygen production, and MCP1 production in murine lupus nephritis. PLoS ONE 8, e64650 (2013).
Thacker, S. G. et al. The detrimental effects of IFN-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J. Immunol. 185, 4457–4469 (2010).
Kahlenberg, J. M. et al. An essential role of caspase 1 in the induction of murine lupus and its associated vascular damage. Arthritis Rheumatol. 66, 152–162 (2014).
Shoji, K., Tanaka, T. & Nangaku, M. Role of hypoxia in progressive chronic kidney disease and implications for therapy. Curr. Opin. Nephrol. Hypertens. 23, 161–168 (2014).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Tran, M. et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Invest. 121, 4003–4014 (2011).
Duffield, J. S. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Invest. 124, 2299–2306 (2014).
Falke, L. L., Gholizadeh, S., Goldschmeding, R., Kok, R. J. & Nguyen, T. Q. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat. Rev. Nephrol. 11, 233–244 (2015).
Kok, H. M., Falke, L. L., Goldschmeding, R. & Nguyen, T. Q. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat. Rev. Nephrol. 10, 700–711 (2014).
Ostendorf, T., Eitner, F. & Floege, J. The PDGF family in renal fibrosis. Pediatr. Nephrol. 27, 1041–1050 (2012).
Kaissling, B., Lehir, M. & Kriz, W. Renal epithelial injury and fibrosis. Biochim. Biophys. Acta 1832, 931–939 (2013).
Eddy, A. A. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int. 4, 2–8 (2014).
Van Linthout, S., Miteva, K. & Tschope, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 102, 258–269 (2014).
Tampe, B. & Zeisberg, M. Contribution of genetics and epigenetics to progression of kidney fibrosis. Nephrol. Dial. Transplant. 29, iv72–iv79 (2014).
Dressler, G. R. & Patel, S. R. Epigenetics in kidney development and renal disease. Transl. Res. 165, 166–176 (2015).
Bechtel, W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 16, 544–550 (2010).
Lai, J. Y. et al. MicroRNA-21 in glomerular injury. J. Am. Soc. Nephrol. 26, 805–816 (2015).
Duffield, J. S., Grafals, M. & Portilla, D. MicroRNAs are potential therapeutic targets in fibrosing kidney disease: lessons from animal models. Drug Discov. Today Dis. Models 10, e127–e135 (2013).
Contreras, G. et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int. 69, 1846–1851 (2006).
Isenberg, D. et al. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology (Oxford) 49, 128–140 (2010).
Tamirou, F. et al. Long-term follow-up of the MAINTAIN Nephritis Trial, comparing azathioprine andmycophenolate mofetil as maintenance therapy of lupus nephritis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2014-206897.
Dall'Era, M. et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis Cohort. Arthritis Rheumatol. 67, 1305–1313 (2015).
Hsieh, C. et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res. (Hoboken) 63, 865–874 (2011).
Alsuwaida, A. O. Interstitial inflammation and long-term renal outcomes in lupus nephritis. Lupus 22, 1446–1454 (2013).
Esdaile, J. M., Levinton, C., Federgreen, W., Hayslett, J. P. & Kashgarian, M. The clinical and renal biopsy predictors of long-term outcome in lupus nephritis: a study of 87 patients and review of the literature. Q. J. Med. 72, 779–833 (1989).
Yazdany, J. et al. Quality of care for incident lupus nephritis among Medicaid beneficiaries in the United States. Arthritis Care Res. (Hoboken) 66, 617–624 (2014).
Pokroy-Shapira, E., Gelernter, I. & Molad, Y. Evolution of chronic kidney disease in patients with systemic lupus erythematosus over a long-period follow-up: a single-center inception cohort study. Clin. Rheumatol. 33, 649–657 (2014).
Chung, S. A. et al. Lupus nephritis susceptibility loci in women with systemic lupus erythematosus. J. Am. Soc. Nephrol. 25, 2859–2870 (2014).
Caster, D. J. et al. ABIN1 dysfunction as a genetic basis for lupus nephritis. J. Am. Soc. Nephrol. 24, 1743–1754 (2013).
Sanchez, E. et al. Phenotypic associations of genetic susceptibility loci in systemic lupus erythematosus. Ann. Rheum. Dis. 70, 1752–1757 (2011).
Bolin, K. et al. Association of STAT4 polymorphism with severe renal insufficiency in lupus nephritis. PLoS ONE 8, e84450 (2013).
Kim-Howard, X. et al. ITGAM coding variant (rs1143679) influences the risk of renal disease, discoid rash and immunological manifestations in patients with systemic lupus erythematosus with European ancestry. Ann. Rheum. Dis. 69, 1329–1332 (2010).
Liu, K. et al. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. J. Clin. Invest. 119, 911–923 (2009).
Lee, Y. H. & Bae, S. C. Association between the functional ITGAM rs1143679 G/A polymorphism and systemic lupus erythematosus/lupus nephritis or rheumatoid arthritis: an update meta-analysis. Rheumatol. Int. 35, 815–823 (2014).
Dong, C. et al. Fcγ receptor IIIa single-nucleotide polymorphisms and haplotypes affect human IgG binding and are associated with lupus nephritis in African Americans. Arthritis Rheumatol. 66, 1291–1299 (2014).
Freedman, B. I. et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol. 66, 390–396 (2014).
Colares, V. S. et al. MYH9 and APOL1 gene polymorphisms and the risk of CKD in patients with lupus nephritis from an admixture population. PLoS ONE 9, e87716 (2014).
Lin, C. P. et al. Role of MYH9 and APOL1 in African and non-African populations with lupus nephritis. Genes Immun. 13, 232–238 (2012).
Chung, A. C. & Lan, H. Y. MicroRNAs in renal fibrosis. Front. Physiol. 6, 50 (2015).
Rodríguez-Romo, R., Berman, N., Gomez, A. & Bobadilla, N. A. Epigenetic regulation in the acute kidney injury (AKI) to chronic kidney disease transition (CKD). Nephrology (Carlton) http://dx.doi.org/10.1111/nep.12521.
Bomsztyk, K. & Denisenko, O. Epigenetic alterations in acute kidney injury. Semin. Nephrol. 33, 327–340 (2013).
Liu, N. & Zhuang, S. Treatment of chronic kidney diseases with histone deacetylase inhibitors. Front. Physiol. 6, 121 (2015).
Lv, L. L. et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am. J. Physiol. Renal Physiol. 305, F1220–F1227 (2013).
Te, J. L. et al. Identification of unique microRNA signature associated with lupus nephritis. PLoS ONE 5, e10344 (2010).
Peterson, K. S. et al. Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J. Clin. Invest. 113, 1722–1733 (2004).
Berthier, C. C., Kretzler, M. & Davidson, A. From the large scale expression analysis of lupus nephritis to targeted molecular medicine. J. Data Mining Genomics Proteomics 3, 1000123 (2012).
Bethunaickan, R., Berthier, C. C., Zhang, W., Kretzler, M. & Davidson, A. Comparative transcriptional profiling of 3 murine models of SLE nephritis reveals both unique and shared regulatory networks. PLoS ONE 8, e77489 (2013).
Jacob, C. O. et al. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J. Immunol. 177, 2671–2680 (2006).
Ramanujam, M. et al. Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum. 62, 1457–1468 (2010).
Ge, Y. et al. Cgnz1 allele confers kidney resistance to damage preventing progression of immune complex-mediated acute lupus glomerulonephritis. J. Exp. Med. 210, 2387–2401 (2013).
Venkatachalam, M. A., Weinberg, J. M., Kriz, W. & Bidani, A. K. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol. 26, 1765–1776 (2015).
Srisawat, N., Murugan, R. & Kellum, J. A. Repair or progression after AKI: a role for biomarkers? Nephron Clin. Pract. 127, 185–189 (2014).
Zoja, C., Abbate, M. & Remuzzi, G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol. Dial. Transplant. 30, 706–712 (2014).
Schiffer, L. et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J. Immunol. 171, 489–497 (2003).
Kulkarni, O. et al. Anti-Ccl2 Spiegelmer permits 75% dose reduction of cyclophosphamide to control diffuse proliferative lupus nephritis and pneumonitis in MRL-Fas(lpr) mice. J. Pharmacol. Exp. Ther. 328, 371–377 (2009).
Satoskar, A. A. et al. Characterization of glomerular diseases using proteomic analysis of laser capture microdissected glomeruli. Mod. Pathol. 25, 709–721 (2012).
Parikh, S. V., Ayoub, I. & Rovin, B. H. The kidney biopsy in lupus nephritis: time to move beyond histology. Nephrol. Dial. Transplant. 30, 3–6 (2015).
Rovin, B. H. & Klein, J. B. Proteomics and autoimmune kidney disease. Clin. Immunol. http://dx.doi.org/10.1016/j.clim.2015.04.021.
Romick-Rosendale, L. E. et al. Identification of urinary metabolites that distinguish membranous lupus nephritis from proliferative lupus nephritis and focal segmental glomerulosclerosis. Arthritis Res. Ther. 13, R199 (2011).
Nowling, T. K. et al. Renal glycosphingolipid metabolism is dysfunctional in lupus nephritis. J. Am. Soc. Nephrol. 26, 1402–1413 (2014).
Reyes-Thomas, J., Blanco, I. & Putterman, C. Urinary biomarkers in lupus nephritis. Clin. Rev. Allergy Immunol. 40, 138–150 (2011).
Abulaban, K. M. & Brunner, H. I. Biomarkers for childhood-onset systemic lupus erythematosus. Curr. Rheumatol. Rep. 17, 471 (2015).
Davidson, A. & Aranow, C. Lupus nephritis: lessons from murine models. Nat. Rev. Rheumatol. 6, 13–20 (2010).
Corapi, K. M., Dooley, M. A. & Pendergraft, W. F. 3rd. Comparison and evaluation of lupus nephritis response criteria in lupus activity indices and clinical trials. Arthritis Res. Ther. 17, 110 (2015).
Lech, M. et al. NLRP3 and ASC suppress lupus-like autoimmunity by driving the immunosuppressive effects of TGF-β receptor signalling. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2014-205496.
Campbell, A. M., Kashgarian, M. & Shlomchik, M. J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 4, 157ra141 (2012).
Sharma, S. et al. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc. Natl Acad. Sci. USA 112, E710–E717 (2015).
Saxena, V. et al. Dual roles of immunoregulatory cytokine TGF-β in the pathogenesis of autoimmunity-mediated organ damage. J. Immunol. 180, 1903–1912 (2008).
Aringer, M. & Smolen, J. S. Efficacy and safety of TNF-blocker therapy in systemic lupus erythematosus. Expert Opin. Drug Saf. 7, 411–419 (2008).
Liu, Z. & Davidson, A. Taming lupus—a new understanding of pathogenesis is leading to clinical advances. Nat. Med. 18, 871–882 (2012).
Houssiau, F. A. Biologic therapy in lupus nephritis. Nephron Clin. Pract. 128, 255–260 (2014).
Liu, Y. & Anders, H. J. Lupus nephritis: from pathogenesis to targets for biologic treatment. Nephron Clin. Pract. 128, 224–231 (2014).
Chan, T. M. Treatment of severe lupus nephritis: the new horizon. Nat. Rev. Nephrol. 11, 46–61 (2015).
Rovin, B. H. & Parikh, S. V. Lupus nephritis: the evolving role of novel therapeutics. Am. J. Kidney Dis. 63, 677–690 (2014).
Allegretti, M., Cesta, M. C., Garin, A. & Proudfoot, A. E. Current status of chemokine receptor inhibitors in development. Immunol. Lett. 145, 68–78 (2012).
Asquith, D. L., Bryce, S. A. & Nibbs, R. J. Targeting cell migration in rheumatoid arthritis. Curr. Opin. Rheumatol. 27, 204–211 (2015).
Alexander, T. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann. Rheum. Dis. http:dx.doi.org/10.1136/annrheumdis-2014-206016.
Cernaro, V. et al. New therapeutic strategies under development to halt the progression of renal failure. Expert Opin. Investig. Drugs 23, 693–709 (2014).
Sabuda-Widemann, D., Grabensee, B., Schwandt, C. & Blume, C. Mycophenolic acid inhibits the autocrine PDGF-B synthesis and PDGF-BB-induced mRNA expression of Egr-1 in rat mesangial cells. Nephrol. Dial. Transplant. 24, 52–61 (2009).
Chandrashekar, K. & Juncos, L. A. Endothelin antagonists in diabetic nephropathy: back to basics. J. Am. Soc. Nephrol. 25, 869–871 (2014).
Lin, S. L. et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am. J. Pathol. 178, 911–923 (2011).
Tampe, D. & Zeisberg, M. Potential approaches to reverse or repair renal fibrosis. Nat. Rev. Nephrol. 10, 226–237 (2014).
Madsen, D. H. et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J. Cell Biol. 202, 951–966 (2013).
Kawakami, T., Ren, S. & Duffield, J. S. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J. Pathol. 229, 221–231 (2013).
Anders, H. J. & Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 80, 915–925 (2011).
Vielhauer, V., Kulkarni, O., Reichel, C. A. & Anders, H. J. Targeting the recruitment of monocytes and macrophages in renal disease. Semin. Nephrol. 30, 318–333 (2010).
Ardoin, S. et al. An approach to validating criteria for proteinuric flare in systemic lupus erythematosus glomerulonephritis. Arthritis Rheum. 63, 2031–2037 (2011).
Parikh, S. V., Nagaraja, H. N., Hebert, L. & Rovin, B. H. Renal flare as a predictor of incident and progressive CKD in patients with lupus nephritis. Clin. J. Am. Soc. Nephrol. 9, 279–284 (2014).
Vielhauer, V. & Anders, H. J. Chemokines and chemokine receptors as therapeutic targets in chronic kidney disease. Front. Biosci. (Schol Ed) 1, 1–12 (2009).
Acknowledgements
The author's work is supported by NIH R01 DK085241-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Davidson, A. What is damaging the kidney in lupus nephritis?. Nat Rev Rheumatol 12, 143–153 (2016). https://doi.org/10.1038/nrrheum.2015.159
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.159
This article is cited by
-
Urinary neutrophil gelatinase-associated lipocalin (uNGAL) and kidney injury molecule-1 (uKIM-1) as markers of active lupus nephritis
Clinical Rheumatology (2024)
-
The immunoregulatory roles of non-haematopoietic cells in the kidney
Nature Reviews Nephrology (2023)
-
Burden of disease and real-world treatment patterns of patients with systemic lupus erythematosus in the Australian OPAL dataset
Clinical Rheumatology (2023)
-
Persistent inflammation–immunosuppression–catabolism syndrome in patients with systemic lupus erythematosus
International Urology and Nephrology (2023)
-
HSPB5 suppresses renal inflammation and protects lupus-prone NZB/W F1 mice from severe renal damage
Arthritis Research & Therapy (2022)