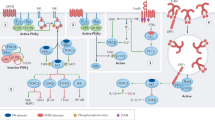
Key Points
-
This review discusses the many protein tyrosine phosphatases (PTPs) that are expressed by T cells and our current understanding of their biology and roles in human disease.
-
T cells express ∼50–60 different PTPs.
-
CD45, SHP2 (SRC homology 2 (SH2)-domain-containing PTP 2), and LMPTP (low-molecular-weight PTP) mainly have positive roles in signalling through the T-cell receptor.
-
SHP1, LYP (lymphoid-specific PTP), PTP-PEST (PTP with PEST (proline-, glutamic-acid-, serine- and threonine-rich) domains), PTP-HSCF (PTP haematopoietic stem-cell fraction) and PTPH1 (PTP H1) are potent negative regulators of T-cell activation.
-
HEPTP (haematopoietic PTP) and many dual-specific PTPs dephosphorylate mitogen-activated protein kinases.
-
PTP-MEG2 (PTP megakaryocyte 2) regulates secretory vesicles.
-
A polymorphism in LYP is associated with autoimmune disease.
-
Some pathogens use PTPs for immune evasion.
Abstract
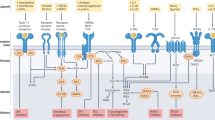
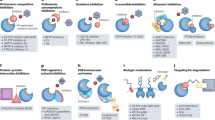
Reversible tyrosine phosphorylation of proteins is a key regulatory mechanism for numerous important aspects of eukaryotic physiology and is catalysed by kinases and phosphatases. Together, cells of the immune system express at least half of the 107 protein tyrosine phosphatase (PTP) genes in the human genome, most of which encode multidomain proteins that contain protein- and phospholipid-interaction domains. Here, we discuss the diverse but specific, and important, roles that PTPs have in immune cells, focusing mainly on T and B cells, and we highlight recent evidence that even subtle alterations in PTPs can cause immune dysfunction and human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hunter, T. & Sefton, B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc. Natl Acad. Sci. USA 77, 1311–1315 (1980).
Mustelin, T., Abraham, R. T., Rudd, C. E., Alonso, A. & Merlo, J. J. Protein tyrosine phosphorylation in T cell signaling. Front. Biosci. 7, d918–d969 (2002).
Mustelin, T. Src Family Tyrosine Kinases in Leukocytes 1–155 (Landes, Austin, 1994).
Mustelin, T., Rahmouni, S., Bottini, N. & Alonso, A. Role of protein tyrosine phosphatases in T cell activation. Immunol. Rev. 191, 139–147 (2003).
Mustelin, T. & Taskén, K. Positive and negative regulation of T cell activation through kinases and phosphatases. Biochem. J. 371, 15–27 (2003).
Alonso, A. et al. Protein tyrosine phosphatases in the human genome. Cell 117, 699–711 (2004). This paper reviews the entire complement of 107 PTP genes in the human genome.
Andersen, J. N. et al. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J. 18, 8–13 (2004).
Samelson, L. E., Patel, M. D., Weissman, A. M., Harford, J. B. & Klausner, R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell 46, 1083–1090 (1986).
Hsi, E. D. et al. T cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J. Biol. Chem. 264, 10836–10842 (1989).
Mustelin, T., Coggeshall, K. M., Isakov, N. & Altman, A. Tyrosine phosphorylation is required for T cell antigen receptor-mediated activation of phospholipase C. Science 247, 1584–1587 (1990).
Saltzman, E. M., Thom, R. R. & Casnellie, J. E. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J. Biol. Chem. 263, 6956–6959 (1988).
Gold, M. R., Matsuuchi, L., Kelly, R. B. & DeFranco, A. L. Tyrosine phosphorylation of components of the B cell antigen receptors following receptor crosslinking. Proc. Natl Acad. Sci. USA 88, 3436–3440 (1991).
Mustelin, T. T cell antigen receptor signaling: three families of tyrosine kinases and a phosphatase. Immunity 1, 351–356 (1994).
Weiss, A. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell 73, 209–212 (1993).
Mustelin, T. et al. Protein tyrosine phosphatases. Front. Biosci. 7, 85–142 (2002).
Iivanainen, A. V., Lindqvist, C., Mustelin, T. & Andersson, L. C. Phosphotyrosine phosphatases are involved in reversion of T lymphoblastic proliferation. Eur. J. Immunol. 20, 2509–2512 (1990).
O'Shea, J. J., McVicar, D. W., Bailey, T. L., Burns, C. & Smyth, M. J. Activation of human peripheral blood T lymphocytes by pharmacological induction of protein-tyrosine phosphorylation. Proc. Natl Acad. Sci. USA 89, 10306–10310 (1992).
Secrist, J. P., Burns, L. A., Karnitz, L., Koretzky, G. A. & Abraham, R. T. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J. Biol. Chem. 268, 5886–5893 (1993). References 17 and 18 were the first papers to show that acute inhibition of PTPs is sufficient to cause T-cell activation.
Oetken., C. et al. Phenylarsine oxide augments tyrosine phosphorylation in hematopoietic cells. Eur. J. Haematol. 49, 208–214 (1992).
Earp, H. S., Austin, K. S., Buessow, S. C. & Gillespie, G. Y. Membranes from T and B lymphocytes have different patterns of tyrosine phosphorylation. Proc. Natl Acad. Sci. USA 81, 2347–2351 (1984).
Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Gjörloff-Wingren, A. et al. Subcellular localization of intracellular protein tyrosine phosphatases in T cells. Eur. J. Immunol. 30, 2412–2421 (2000).
Gjörloff-Wingren, A., Saxena, M., Williams, S., Hammi, D. & Mustelin, T. Characterization of TCR-induced receptor-proximal signaling events negatively regulated by the protein tyrosine phosphatase PEP. Eur. J. Immunol. 29, 3845–3854 (1999).
Han, S., Williams, S. & Mustelin, T. Cytoskeletal protein tyrosine phosphatase PTPH1 reduces T cell antigen receptor signaling. Eur. J. Immunol. 30, 1318–1325 (2000).
Bottini, N. et al. Activation of ZAP-70 through specific dephosphorylation at the inhibitory Tyr-292 by the low molecular weight phosphotyrosine phosphatase (LMPTP). J. Biol. Chem. 277, 24220–24224 (2002).
Townley, R., Shen, S. -H., Banville, D. & Ramachandran, C. Inhibition of the activity of protein tyrosine phosphatase 1C by its SH2 domains. Biochemistry 32, 13414–13418 (1993).
Pei, D., Lorenz, U., Klingmüller, U., Neel, B. G. & Walsh, C. T. Intramolecular regulation of protein tyrosine phosphatase SH-PTP1: a new function for Src homology 2 domains. Biochemistry 33, 15483–15493 (1994).
Zhao, Z., Larocque, R., Ho, W. -T., Fischer, E. H. & Shen, S. -H. Purification and characterization of PTP2C, a widely distributed protein tyrosine phosphatase containing two SH2 domains. J. Biol. Chem. 269, 8780–8785 (1994).
Pei, D., Wang, J. & Walsh, C. T. Differential functions of the two Src homology 2 domains in protein tyrosine phosphatase SH-PTP1. Proc. Natl Acad. Sci. USA 93, 1141–1145 (1996).
Hof, P., Pluskey, S., Dhe-Paganon, S., Eck, M. J. & Shoelson, S. E. Crystal structure of the tyrosine phosphatase SHP-2. Cell 92, 441–450 (1998).
Alonso, A. et al. VHY, a novel, myristoylated testis-restricted dual specificity protein phosphatase related to VHX. J. Biol. Chem. 279, 32586–32591 (2004).
Mustelin, T., Coggeshall, K. M. & Altman, A. Rapid activation of the T cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc. Natl Acad. Sci. USA 86, 6302–6306 (1989). This paper was the first to show that the PTP CD45 can activate the PTK LCK by direct dephosphorylation.
Mustelin, T. & Altman, A. Dephosphorylation and activation of the T cell tyrosine kinase pp56lck by the leukocyte common antigen (CD45). Oncogene 5, 809–813 (1990).
Mustelin, T. et al. Regulation of the p59fyn protein tyrosine kinase by the CD45 phosphotyrosine phosphatase. Eur. J. Immunol. 22, 1173–1178 (1992).
Brockdorff, J., Williams, S., Couture, C. & Mustelin, T. Dephosphorylation of ZAP-70 and inhibition of T cell activation by activated SHP1. Eur. J. Immunol. 29, 2539–2550 (1999).
Zheng, X. M., Wang, Y. & Pallen, C. J. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature 359, 336–339 (1992).
Mustelin, T. & Hunter, T. Meeting at mitosis: cell cycle-specific regulation of c-Src by RPTPα. Sci. STKE [online] 2002, PE3 (2002).
Salmeen, A. et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423, 769–773 (2003).
van Montfort, R. L. M., Congreve, M., Tisi, D., Carr, R. & Jhoti, H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature 423, 773–777 (2003). References 38 and 39 report the crystallization of oxidized PTP1B and reveal that oxidation of the catalytic cysteine residue results in the formation of a sulphenyl amide moiety, which is resistant to further oxidation (which would be irreversible) and can be reduced back to the catalytically active free cysteinyl.
Meng, T. -C., Buckley, D. A., Galic, S., Tiganis, T. & Tonks, N. K. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J. Biol. Chem. 279, 37716–37725 (2004).
Wang, X. et al. The tumor suppressor PTEN regulates T cell survival and antigen receptor signaling by acting as a phosphatidylinositol 3-phosphatase. J. Immunol. 164, 1934–1939 (2000).
Qu, C. -K., Nguyen, S., Chen, J. & Feng, G. -S. Requirement of Shp-2 tyrosine phosphatase in lymphoid and hematopoietic cell development. Blood 97, 911–914 (2001).
Kishihara, K. et al. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell 74, 143–156 (1993).
Gronda, M., Arab, S., Iafrate, B., Suzuki, H. & Zanke, B. Hematopoietic protein tyrosine phosphatase suppresses extracellular stimulus-regulated kinase activation. Mol. Cell. Biol. 21, 6851–6858 (2001).
Elchebly, M. et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283, 1544–1548 (1999).
You-Ten, K. E. et al. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 186, 683–693 (1997).
Charbonneau, H., Tonks, N. K., Walsh, K. A. & Fischer, E. H. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc. Natl Acad. Sci. USA 85, 7182–7186 (1988).
Tonks, N. K., Charbonneau, H., Diltz, C. D., Fischer, E. H. & Walsh, K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry 27, 8695–8701 (1988). Although reference 47 was the first to report that CD45 has a high degree of homology with the first identified PTP, PTP1B, and therefore was likely to be a PTP itself, reference 48 directly showed this by providing evidence that CD45 has tyrosine-phosphatase activity.
Pingel, J. T. & Thomas, M. L. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell 58, 1055–1065 (1989). This paper was the first to show that expression of CD45 is important for T-cell activation.
Hermiston, M. L., Xu, Z. & Weiss, A. CD45: a critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 21, 107–137 (2003).
Koretzky, G. A., Picus, J., Thomas, M. L. & Weiss, A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature 346, 66–68 (1990).
Volarevic, S. et al. The CD45 tyrosine phosphatase regulates phosphotyrosine homeostasis and its loss reveals a novel pattern of late T cell receptor-induced Ca2+ oscillations. J. Exp. Med. 176, 835–844 (1992).
Ostergaard, H. L. et al. Expression of CD45 alters phosphorylation of the Lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc. Natl Acad. Sci. USA 86, 8959–8963 (1989).
Seavitt, J. et al. Expression of the p56lck Y505F mutation in CD45-deficient mice rescues thymocyte development. Mol. Cell. Biol. 19, 4200–4208 (1999).
Mustelin, T. & Burn, P. Regulation of src family tyrosine kinases in lymphocytes. Trends Biochem. Sci. 18, 215–220 (1993).
Davidson, D., Bakinowski, M., Thomas, M. L., Horejsi, V. & Veillette, A. Phosphorylation-dependent regulation of T cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol. Cell. Biol. 23, 2017–2025 (2003).
Kawabuchi, M. et al. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 404, 999–1003 (2000).
Brdicka, T. et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adapter protein, binds the protein tyrosine kinase Csk and is involved in regulation of T cell activation. J. Exp. Med. 191, 1591–1604 (2000).
Torgersen, K. M. et al. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J. Biol. Chem. 276, 29313–29318 (2001).
Vang, T., Abrahamsen, H., Myklebust, S., Horejsi, V. & Taskén, K. Combined spatial and enzymatic regulation of Csk by cAMP and protein kinase A inhibits T cell receptor signaling. J. Biol. Chem. 278, 17597–17604 (2003).
Vang, T. et al. Knockdown of C-terminal Src kinase by siRNA-mediated RNA interference augments T cell receptor signaling in mature T cells. Eur. J. Immunol. 34, 2191–2199 (2004).
Zhang, S. Q. et al. Shp2 regulates Src family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell 13, 341–351 (2004).
Ledbetter, J. A., Tonks, N. K., Fischer, E. H. & Clark, E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc. Natl Acad. Sci. USA 85, 8628–8633 (1988).
Kiener, P. A. & Mittler, R. S. CD45-protein tyrosine phosphatase cross-linking inhibits T cell receptor CD3-mediated activation in human T cells. J. Immunol. 143, 23–28 (1989).
Ostergaard, H. L. & Trowbridge, I. S. Coclustering of CD45 with CD4 or CD8 alters the phosphorylation and kinase activity of p56lck. J. Exp. Med. 172, 347–350 (1990).
Volarevic, S. et al. Regulation of TCR signaling by CD45 lacking transmembrane and extracellular domains. Science 260, 541–544 (1993).
Furukawa, T., Itoh, M., Krueger, N. X., Streuli, M. & Saito, H. Specific interaction of the CD45 protein-tyrosine phosphatase with tyrosine phosphorylated CD3 ζ chain. Proc. Natl Acad. Sci. USA 91, 10928–10932 (1994).
Mustelin, T. et al. Regulation of the p70zap tyrosine protein kinase by the CD45 phosphotyrosine phosphatase in T cells. Eur. J. Immunol. 25, 942–946 (1995).
D'Oro, U., Sakaguchi, K., Appella, E. & Ashwell, J. D. Mutational analysis of Lck in CD45-negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Mol. Cell. Biol. 16, 4996–5003 (1996).
Katagiri, T. et al. CD45 negatively regulates Lyn activity by dephosphorylating both positive and negative regulatory tyrosine residues in immature B cells. J. Immunol. 163, 1321–1330 (1999).
He, X., Woodford-Thomas, T. A., Johnson, K. G., Shah, D. D. & Thomas, M. L. Targeting of CD45 protein tyrosine phosphatase activity to lipid microdomains on the T cell surface inhibits TCR signaling. Eur. J. Immunol. 32, 2578–2584 (2002).
Montixi, C. et al. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17, 5334–5340 (1998).
Edmonds, S. D. & Ostergaard, H. L. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J. Immunol. 169, 5036–5046 (2002).
Irles, C. et al. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nature Immunol. 4, 189–192 (2003).
Freiberg, B. A. et al. Staging and resetting T cell activation in SMACs. Nature Immunol. 3, 911–916 (2002).
Majeti, R., Bilwes, A. M., Noel, J. P., Hunter, T. & Weiss, A. Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge. Science 279, 88–91 (1998).
Majeti, R. et al. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell 103, 1059–1069 (2000).
Xu, Z. & Weiss, A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nature Immunol. 3, 764–770 (2002).
Dianzani, U. et al. Isoform-specific associations of CD45 with accessory molecules in human T lymphocytes. Eur. J. Immunol. 22, 365–371 (1992).
Matthews, R. J., Bowne, D. B., Flores, E. & Thomas, M. L. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol. Cell. Biol. 12, 2396–2405 (1992).
Vivier, E. & Daëron, M. Immunoreceptor tyrosine-based inhibition motifs. Immunol. Today 18, 286–291 (1997).
Mustelin, T. et al. T cell activation: the coming of the phosphatases. Front. Biosci. 3, D1060–D1096 (1998).
Chiang, G. G. & Sefton, B. M. Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr-394 by the SHP-1 protein-tyrosine phosphatase. J. Biol. Chem. 276, 23173–23179 (2001).
Raab, M. & Rudd, C. E. Hematopoietic cell phosphatase (HCP) regulates p56LCK phosphorylation and ZAP-70 binding to T cell receptor ζ chain. Biochem. Biophys. Res. Commun. 222, 50–57 (1996).
Tsui, H. W., Siminovitch, K. A., de Souza, L. & Tsui, F. W. L. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nature Genet. 4, 124–129 (1993).
Shultz, L. D. & Green, M. C. Motheaten, an immunodeficient mutant of the mouse. II. Depressed immune competence and elevated serum immunoglobulins. J. Immunol. 116, 936–943 (1976).
Pani, G., Fischer, K. -D., Mlinaric-Rascan, I. & Siminovitch, K. A. Signaling capacity of the T cell antigen receptor is negatively regulated by the PTP1C tyrosine phosphatase. J. Exp. Med. 184, 839–852 (1996).
Sathish, J. G. et al. CD22 is a functional ligand for SHP1 in primary T cells. J. Biol. Chem. 279, 47783–47791 (2004).
Roy, G., Matthews, J., Woodford-Thomas, T. & Thomas, M. L. The function of protein tyrosine phosphatases in immune regulation. Adv. Protein Phosphatases 9, 121–138 (1995).
Cloutier, J. -F. & Veillette, A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 15, 4909–4918 (1996).
Cloutier, J. -F. & Veillette, A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J. Exp. Med. 189, 111–121 (1999). References 90 and 91 document the high-stoichiometry association between the PEP phosphatase and the inhibitory kinase CSK. The papers conclude that the interaction between the two enzymes helps PEP reach its SRC-family PTK targets.
Cohen, S., Dadi, H., Shaoul, E., Sharfe, N. & Roifman, C. M. Cloning and characterization of a lymphoid-specific, inducible human protein tyrosine phosphatase, Lyp. Blood 93, 2013–2024 (1999).
Bottini, N. et al. A functional variant of lymphoid tyrosine phosphatase is associated with type 1 diabetes. Nature Genet. 36, 337–338 (2004). This paper reports that a single amino-acid substitution at position 620 of the human PTP LYP is sufficient to cause a marked increase in the incidence of type 1 diabetes. This increase correlates with a loss of association with CSK.
Begovich, A. B. et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 75, 330–337 (2004).
Kyogoku, C. et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am. J. Hum. Genet. 75, 504–507 (2004).
Smyth, D. et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes 53, 3020–3023 (2004).
Hasegawa, K. et al. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science 303, 685–689 (2004). This paper describes the defects in T-cell immunity that are found in Pep−/− mice.
Davidson, D., Cloutier, J. F., Gregorieff, A. & Veillette, A. Inhibitory tyrosine protein kinase p50csk is associated with protein-tyrosine phosphatase PTP-PEST in hemopoietic and non-hemopoietic cells. J. Biol. Chem. 272, 23455–23462 (1997).
Wang, B., Lemay, S., Tsai, S. & Veillette, A. SH2 domain-mediated interaction of inhibitory protein tyrosine kinase Csk with protein tyrosine phosphatase-HSCF. Mol. Cell. Biol. 21, 1077–1088 (2001).
Cote, J. F., Charest, A., Wagner, J. & Tremblay, M. L. Combination of gene targeting and substrate trapping to identify substrates of protein tyrosine phosphatases using PTP-PEST as a model. Biochemistry 37, 13128–13137 (1998).
Garton, A. J., Flint, A. J. & Tonks, N. K. Identification of p130cas as a substrate for the cytosolic protein tyrosine phosphatase PTP-PEST. Mol. Cell. Biol. 16, 6408–6418 (1996).
Davidson, D. & Veillette, A. PTP-PEST, a scaffold protein tyrosine phosphatase, negatively regulates lymphocyte activation by targeting a unique set of substrates. EMBO J. 20, 3414–3426 (2001).
Spencer, S. et al. PSTPIP: a tyrosine phosphorylated cleavage furrow-associated protein that is a substrate for a PEST tyrosine phosphatase. J. Cell Biol. 138, 845–860 (1997).
Sozio, M. S. et al. PTPH1 is a predominant protein-tyrosine phosphatase capable of interacting with and dephosphorylating the T cell receptor ζ subunit. J. Biol. Chem. 279, 7760–7768 (2004).
Zhang, S. -H., Liu, J., Kobayashi, R. & Tonks, N. K. Identification of the cell cycle regulator VCP (p97/CDC48) as a substrate of the band 4.1-related protein-tyrosine phosphatase PTPH1. J. Biol. Chem. 274, 17806–17812 (1999).
Egerton, M. et al. VCP, the mammalian homolog of CDC48, is tyrosine phosphorylated in response to T cell antigen receptor activation. EMBO J. 11, 3533–3540 (1992).
Lavoie, C. et al. Tyrosine phosphorylation of p97 regulates transitional endoplasmic reticulum assembly in vitro. Proc. Natl Acad. Sci. USA 97, 13637–13642 (2000).
Zheng, Y., Schlondorff, J. & Blobel, C. P. Evidence for regulation of the tumor necrosis factor α-convertase (TACE) by protein-tyrosine phosphatase PTPH1. J. Biol. Chem. 277, 42463–42470 (2002).
Tailor, P., Williams, S., Gilman, J., Couture, C. & Mustelin, T. Regulation of the low molecular weight phosphotyrosine phosphatase (LMPTP) by phosphorylation at tyrosines 131 and 132. J. Biol. Chem. 272, 5371–5376 (1997).
Alonso, A., Saxena, M., Williams, S. & Mustelin, T. Inhibitory role for dual-specificity phosphatase VHR in T cell antigen receptor and CD28-induced Erk and Jnk activation. J. Biol. Chem. 276, 4766–4771 (2001).
Alonso, A. et al. Tyrosine phosphorylation of VHR phosphatase by ZAP-70. Nature Immunol. 4, 44–48 (2003).
Saxena, M. & Mustelin, T. Extracellular signals and scores of phosphatases: all roads lead to MAP kinase. Semin. Immunol. 12, 387–396 (2000).
Saxena, M., Williams, S., Gilman, J. & Mustelin, T. Negative regulation of T cell antigen receptor signaling by hematopoietic tyrosine phosphatase (HePTP). J. Biol. Chem. 273, 15340–15344 (1998).
Saxena, M., Williams, S., Brockdorff, J., Gilman, J. & Mustelin, T. Inhibition of T cell signaling by MAP kinase-targeted hematopoietic tyrosine phosphatase (HePTP). J. Biol. Chem. 274, 11693–11700 (1999).
Saxena, M., Williams, S., Taskén, K. & Mustelin, T. Crosstalk between cAMP-dependent kinase and MAP kinase through hematopoietic protein tyrosine phosphatase (HePTP). Nature Cell Biol. 1, 305–311 (1999).
Nika, K. et al. Hematopoietic protein tyrosine phosphatase (HePTP) phosphorylation by cAMP-dependent protein kinase in T cells: dynamics and subcellular location. Biochem. J. 378, 335–342 (2004).
Klingmüller, U., Lorenz, U., Cantley, L. C., Neel, B. G. & Lodish, H. F. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 80, 729–738 (1995).
Myers, M. P. et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276, 47771–47774 (2001).
ten Hoeve, J. et al. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol. Cell. Biol. 22, 5662–5668 (2002).
Zhu, W., Mustelin, T. & David, M. Arginine methylation of STAT1 regulates its dephosphorylation by TcPTP. J. Biol. Chem. 277, 35787–35790 (2002).
Wang, X. et al. Enlargement of secretory vesicles by protein tyrosine phosphatase PTP-MEG2 in RBL mast cells and Jurkat T cells. J. Immunol. 168, 4612–4619 (2002).
Huynh, H. et al. Homotypic secretory vesicle fusion induced by the protein tyrosine phosphatase MEG2 depends on polyphosphoinositides in T cells. J. Immunol. 171, 6661–6671 (2003).
Huynh, H. et al. Control of vesicle fusion by a tyrosine phosphatase. Nature Cell Biol. 6, 831–839 (2004).
Matsushita, M., Tsuchiya, N., Oka, T., Yamane, A. & Tokunaga, K. New variations of human SHP-1. Immunogenetics 49, 577–579 (1999).
Jury, E. C., Kabouridis, P. S., Flores-Borja, F., Mageed, R. A. & Isenberg, D. A. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J. Clin. Invest. 113, 1176–1187 (2004).
Tchilian, E. Z. et al. A deletion in the gene encoding the CD45 antigen in a patient with SCID. J. Immunol. 166, 1308–1313 (2001).
Jacobsen, M. et al. A point mutation in PTPRC is associated with the development of multiple sclerosis. Nature Genet. 26, 495–499 (2000).
Barcellos, L. F. et al. PTPRC (CD45) is not associated with the development of multiple sclerosis in U.S. patients. Nature Genet. 29, 23–24 (2001).
Vorechovsky, I. et al. Does 77C→G in PTPRC modify autoimmune disorders linked to the major histocompatibility locus? Nature Genet. 29, 22–23 (2001).
Bottini, N., Bottini, E., Gloria-Bottini, F. & Mustelin, T. Low-molecular-weight protein tyrosine phosphatase and human disease: in search of biochemical mechanisms. Arch. Immunol. Ther. Exp. (Warsz.) 50, 95–104 (2002).
Bottini, N. et al. Genetic control of serum IgE levels: a study of low molecular weight protein tyrosine phosphatase. Clin. Genet. 63, 228–231 (2003).
Cornelis, G. R. & Wolf-Watz, H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23, 861–867 (1997).
Andersson, K. et al. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol. Microbiol. 20, 1057–1069 (1996).
Yao, T., Mecsas, J., Healy, J. I., Falkow, S. & Chien, Y. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, YopH. J. Exp. Med. 190, 1343–1350 (1999).
Alonso, A. et al. Lck dephosphorylation at Y394 and inhibition of T cell antigen receptor signaling by Yersinia phosphatase YopH. J. Biol. Chem. 279, 4922–4928 (2004). References 134 and 135 show that the phosphatase YopH from Yersinia spp. is a potent inhibitor of T-cell activation. Reference 135 shows that the molecular mechanism of inhibition involves the dephosphorylation of Y394 of LCK, resulting in complete abrogation of signalling through the TCR.
Murli, S., Watson, R. O. & Galan, J. E. Role of tyrosine kinases and the tyrosine phosphatase SptP in the interaction of Salmonella with host cells. Cell. Microbiol. 3, 795–810 (2001).
Singh, R. et al. Disruption of MptpB impairs the ability of Mycobacterium tuberculosis to survive in guinea pigs. Mol. Microbiol. 50, 751–762 (2003).
Lazo, J. S. & Wipf, P. Small molecule regulation of phosphatase-dependent cell signaling pathways. Oncol. Res. 13, 347–352 (2003).
Liang, F. et al. Aurintricarboxylic acid blocks in vitro and in vivo activity of YopH, an essential virulent factor of Yersinia pestis, the agent of the plague. J. Biol. Chem. 278, 41734–41741 (2003).
Acknowledgements
We apologize for being unable (owing to space limitations) to cite many important contributions made by our colleagues in the field. T.M. is supported by the National Institutes of Health, United States. T.V. is supported by The Norwegian Cancer Society. N.B. is supported by the American Italian Cancer Foundation, United States, and The Litta Foundation, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- POLYCLONALLY ACTIVATED
-
Activation of all T cells by mitogens in an antigen-independent manner.
- BLASTOID MORPHOLOGY
-
The larger size and appearance of an activated T cell.
- SRC FAMILY
-
A group of structurally related cytoplasmic and/or membrane-associated enzymes that are named after the prototypical member, SRC. In haematopoietic cells, SRC kinases — such as LCK, FYN and LYN — are the first protein tyrosine kinases that are activated after stimulation through the immunoreceptors. They phosphorylate ITAMs (immunoreceptor tyrosine-based activation motifs) that are present in the signal-transducing subunits of the immunoreceptors, thereby providing binding sites for SRC homology 2 (SH2)-domain-containing molecules, such as SYK (spleen tyrosine kinase).
- SRC HOMOLOGY 2 DOMAIN
-
(SH2 domain). A protein domain that is commonly found in signal-transduction molecules. It specifically recognizes phosphotyrosine-containing peptide sequences in proteins.
- SH3 DOMAIN
-
(SRC homology 3 domain). A protein domain that is commonly found in signal-transduction molecules. It specifically interacts with certain proline-containing peptides. Classically, it contains either (R/K)XXPXXP or PXXPXR motifs, where X denotes any amino acid.
- ERM DOMAIN
-
A protein–protein interaction domain found in ezrin, radixin and moesin.
- N-LINKED CARBOHYDRATES
-
Sugars that are attached to asparagine residues of proteins.
- O-LINKED CARBOHYDRATES
-
Sugars that are attached to serine and/or threonine residues of proteins.
- MYRISTOYLATION
-
The covalent attachment of a hydrophobic myristoyl group to the amino-terminal glycine residue of a nascent polypeptide.
- FARNESYLATION
-
The covalent addition of farnesyl (15 carbon) isoprenoids to proteins at cysteine residues by farnesyl transferases, which leads to their constitutive association with the plasma membrane.
- LIPID RAFT
-
Area of the plasma membrane that is rich in cholesterol, glycosphingolipids, several signalling proteins (such as SRC kinases, RAS, LAT and PAG) and glycosylphosphatidylinositol-anchored proteins. Also known as glycolipid-enriched membrane domains (GEMs) and detergent-insoluble glycolipid-enriched membranes (DIGs).
- FLUORESCENCE RESONANCE ENERGY TRANSFER
-
(FRET). This is used to measure protein–protein interactions microscopically or by a FACS (fluorescence-activated cell sorter)-based method. Proteins fused to cyan, yellow or red fluorescent proteins are expressed and assessed for interaction by measuring the energy transfer between fluorophores, which can only occur if proteins physically interact. FRET can also be used to examine the activation state of certain proteins if their activation results in specific protein–protein interactions.
- cSMAC
-
(Central supramolecular activation cluster). The central region of the immune synapse.
- RNA INTERFERENCE
-
(RNAi). The use of double-stranded RNAs with sequences that precisely match a given gene to 'knock-down' the expression of that gene by directing RNA-degrading enzymes to destroy the encoded mRNA transcript.
- FOCAL ADHESION PLAQUE
-
The closest contact site of a cell with its environment, which is formed by integrin clustering. The integrins link the extracellular environment to the actin cytoskeleton by a complex assembly of adaptor proteins.
- PDZ DOMAIN
-
(PSD95, DLGA and ZO1 homology domain). A protein domain that is commonly found in signal-transduction molecules. It can interact with different amino-acid motifs that are present at the carboxyl terminus of proteins. PDZ domains can also interact with other PDZ domains, with certain internal peptide sequences and even with lipids.
Rights and permissions
About this article
Cite this article
Mustelin, T., Vang, T. & Bottini, N. Protein tyrosine phosphatases and the immune response. Nat Rev Immunol 5, 43–57 (2005). https://doi.org/10.1038/nri1530
Issue Date:
DOI: https://doi.org/10.1038/nri1530
This article is cited by
-
Identification and verification of PTPN3 as a novel biomarker in predicting cancer prognosis, immunity, and immunotherapeutic efficacy
European Journal of Medical Research (2024)
-
Dusp26 phosphatase regulates mitochondrial respiration and oxidative stress and protects neuronal cell death
Cellular and Molecular Life Sciences (2022)
-
Parasite protein phosphatases: biological function, virulence, and host immune evasion
Parasitology Research (2021)
-
Low molecular weight protein tyrosine phosphatase as signaling hub of cancer hallmarks
Cellular and Molecular Life Sciences (2021)
-
Heterozygous Meg2 Ablation Causes Intraocular Pressure Elevation and Progressive Glaucomatous Neurodegeneration
Molecular Neurobiology (2019)