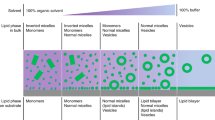
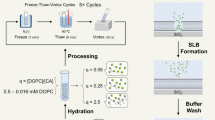
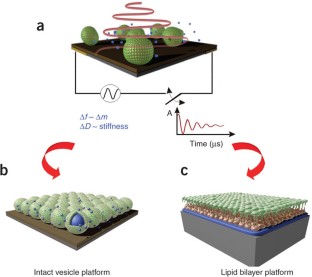
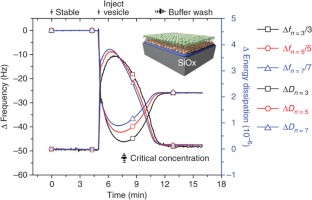
Abstract
Supported lipid bilayers (SLBs) mimic biological membranes and are a versatile platform for a wide range of biophysical research fields including lipid–protein interactions, protein–protein interactions and membrane-based biosensors. The quartz crystal microbalance with dissipation monitoring (QCM-D) has had a pivotal role in understanding SLB formation on various substrates. As shown by its real-time kinetic monitoring of SLB formation, QCM-D can probe the dynamics of biomacromolecular interactions. We present a protocol for constructing zwitterionic SLBs supported on silicon oxide and titanium oxide, and discuss technical issues that need to be considered when working with charged lipid compositions. Furthermore, we explain a recently developed strategy that uses an amphipathic, α-helical (AH) peptide to form SLBs on gold and titanium oxide substrates. The protocols can be completed in less than 3 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
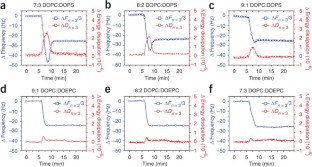
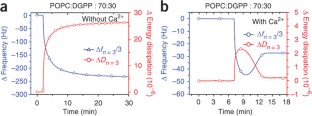
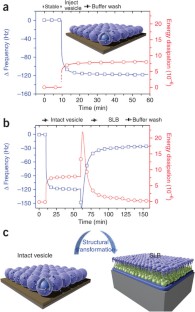
Similar content being viewed by others
References
Sackmann, E. Supported membranes: scientific and practical applications. Science 271, 43–48 (1996).
Tanaka, M. & Sackmann, E. Supported membranes as biofunctional interfaces and smart biosensor platforms. Physica Status Solidi A 203, 3452–3462 (2006).
Hook, F., Kasemo, B., Grunze, M. & Zauscher, S. Quantitative biological surface science: challenges and recent advances. ACS Nano 2, 2428–2436 (2008).
White, R.J. et al. Ionic conductivity of the aqueous layer separating a lipid bilayer membrane and a glass support. Langmuir 22, 10777–10783 (2006).
White, R.J. et al. Single ion-channel recordings using glass nanopore membranes. J. Am. Chem. Soc. 129, 11766–11775 (2007).
Boxer, S.G. Molecular transport and organization in supported lipid membranes. Curr. Opin. Chem. Biol. 4, 704–709 (2000).
Kaiser, H.J. et al. Order of lipid phases in model and plasma membranes. Proc. Natl. Acad. Sci. USA 106, 16645–16650 (2009).
Hook, F., Rodahl, M., Kasemo, B. & Brzezinski, P. Structural changes in hemoglobin during adsorption to solid surfaces: effects of pH, ionic strength, and ligand binding. Proc. Natl. Acad. Sci. USA 95, 12271–12276 (1998).
Hook, F. et al. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: a quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem. 73, 5796–5804 (2001).
Cho, N.J., Cheong, K.H., Lee, C., Frank, C.W. & Glenn, J.S. Binding dynamics of hepatitis C virus′ NS5A amphipathic peptide to cell and model membranes. J. Virol. 81, 6682–6689 (2007).
Cho, N.J., Cho, S.J., Cheong, K.H., Glenn, J.S. & Frank, C.W. Employing an amphipathic viral peptide to create a lipid bilayer on Au and TiO2. J. Am. Chem. Soc. 129, 10050–10051 (2007).
Cooper, M.A. & Singleton, V.T. A survey of the 2001 to 2005 quartz crystal microbalance biosensor literature: applications of acoustic physics to the analysis of biomolecular interactions. J. Mol. Recognit. 20, 154–184 (2007).
Kasemo, B. & Hook, F. Protein and vesicle interaction with surfaces. Abstr. Pap. Am. Chem. Soc. 223, U444–U444 (2002).
Purrucker, O., Fortig, A., Jordan, R., Sackmann, E. & Tanaka, M. Control of frictional coupling of transmembrane cell receptors in model cell membranes with linear polymer spacers. Phys. Rev. Lett. 98 (2007).
Thid, D. et al. Supported phospholipid bilayers as a platform for neural progenitor cell culture. J. Biomed. Mater. Res. 84, 940–953 (2008).
Tu, R.S. & Tirrell, M. Bottom-up design of biomimetic assemblies. Adv. Drug Deliv. Rev. 56, 1537–1563 (2004).
Tamm, L.K. & McConnell, H.M. Supported phospholipid bilayers. Biophys. J. 47, 105–113 (1985).
Axelrod, D., Koppel, D.E., Schlessinger, J., Elson, E. & Webb, W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, 1055–1069 (1976).
Richter, R., Mukhopadhyay, A. & Brisson, A. Pathways of lipid vesicle deposition on solid surfaces: a combined QCM-D and AFM study. Biophys. J. 85, 3035–3047 (2003).
Morigaki, K. & Tawa, K. Vesicle fusion studied by surface plasmon resonance and surface plasmon fluorescence spectroscopy. Biophys. J. 91, 1380–1387 (2006).
Reimhult, E., Larsson, C., Kasemo, B. & Hook, F. Simultaneous surface plasmon resonance and quartz crystal microbalance with dissipation monitoring measurements of biomolecular adsorption events involving structural transformations and variations in coupled water. Anal. Chem. 76, 7211–7220 (2004).
Oxhamre, C., Richter-Dahlfors, A., Zhdanov, V.P. & Kasemo, B. A minimal generic model of bacteria-induced intracellular Ca2+ oscillations in epithelial cells. Biophys. J. 88, 2976–2981 (2005).
Stroumpoulis, D., Parra, A. & Tirrell, M. A kinetic study of vesicle fusion on silicon dioxide surfaces by ellipsometry. AIChE J. 52, 2931–2937 (2006).
Cho, N.J. et al. Alpha-helical peptide-induced vesicle rupture revealing new insight into the vesicle fusion process as monitored in situ by quartz crystal microbalance-dissipation and reflectometry. Anal. Chem. 81, 4752–4761 (2009).
Wang, G. et al. A combined reflectometry and quartz crystal microbalance with dissipation setup for surface interaction studies. Rev. Sci. Instrum. 79, 075107 (2008).
Anderson, T.H. et al. Formation of supported bilayers on silica substrates. Langmuir 25, 6997–7005 (2009).
Kanazawa, K. & Gordon, J. The oscillation frequency of a quartz resonator in contact with a liquid. Anal. Chim. Acta 175, 99–106 (1985).
Kanazawa, K.K. & Reed, C.E. A new description for the viscoelastically loaded quartz resonator. Abstr. Pap. Am. Chem. Soc. 198, 89 Anyl (1989).
Rodahl, M. et al. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discuss. 107, 229–246 (1997).
Rodahl, M., Hook, F. & Kasemo, B. QCM operation in liquids: an explanation of measured variations in frequency and Q factor with liquid conductivity. Anal. Chem. 68, 2219–2227 (1996).
Rodahl, M., Hook, F., Krozer, A., Brzezinski, P. & Kasemo, B. Quartz-crystal microbalance setup for frequency and q-factor measurements in gaseous and liquid environments. Rev. Sci. Instrum. 66, 3924–3930 (1995).
Rodahl, M. & Kasemo, B. Frequency and dissipation-factor responses to localized liquid deposits on a QCM electrode. Sens. Actuators B Chem. 37, 111–116 (1996).
Rodahl, M. & Kasemo, B. On the measurement of thin liquid overlayers with the quartz-crystal microbalance. Sens. Actuators A Phys. 54, 448–456 (1996).
Rodahl, M. & Kasemo, B. A simple setup to simultaneously measure the resonant frequency and the absolute dissipation factor of a quartz crystal microbalance. Rev. Sci. Instrum. 67, 3238–3241 (1996).
Cho, N.J., Kanazawa, K.K., Glenn, J.S. & Frank, C.W. Employing two different quartz crystal microbalance models to study changes in viscoelastic behavior upon transformation of lipid vesicles to a bilayer on a gold surface. Anal. Chem. 79, 7027–7035 (2007).
Hook, F. & Kasemo, B. The QCM-D technique for probing biomacromolecular recognition reactions. Springer Ser. Chem. Sens. Biosens. 5, 425–447 (2007).
Keller, C.A. & Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 75, 1397–1402 (1998).
Keller, C.A., Glasmastar, K., Zhdanov, V.P. & Kasemo, B. Formation of supported membranes from vesicles. Phys. Rev. Lett. 84, 5443–5446 (2000).
Lee, S.E. et al. Biologically functional cationic phospholipid-gold nanoplasmonic carriers of RNA. J. Am. Chem. Soc. 131, 14066–14074 (2009).
Larsson, E.M., Edvardsson, M.E., Langhammer, C., Zoric, I. & Kasemo, B. A combined nanoplasmonic and electrodeless quartz crystal microbalance setup. Rev. Sci. Instrum. 80, 125105 (2009).
Jonsson, M.P., Jonsson, P. & Hook, F. Simultaneous nanoplasmonic and quartz crystal microbalance sensing: analysis of biomolecular conformational changes and quantification of the bound molecular mass. Anal. Chem. 80, 7988–7995 (2008).
Jonsson, M.P., Jonsson, P., Dahlin, A.B. & Hook, F. Supported lipid bilayer formation and lipid-membrane-mediated biorecognition reactions studied with a new nanoplasmonic sensor template. Nano Lett. 7, 3462–3468 (2007).
Misra, N. et al. Bioelectronic silicon nanowire devices using functional membrane proteins. Proc. Natl Acad. Sci. USA 106, 13780–13784 (2009).
Kasemo, B. & Lausmaa, J. Material-tissue interfaces: the role of surface properties and processes. Environ. Health Perspect. 102 (Suppl 5): 41–45 (1994).
Seifert, U., Berndl, K. & Lipowsky, R. Shape transformations of vesicles—phase-diagram for spontaneous-curvature and bilayer-coupling models. Phys. Rev. A 44, 1182–1202 (1991).
Shillcock, J.C. & Lipowsky, R. Tension-induced fusion of bilayer membranes and vesicles. Nat. Mater. 4, 225–228 (2005).
Polozov, I.V., Anantharamaiah, G.M., Segrest, J.P. & Epand, R.M. Osmotically induced membrane tension modulates membrane permeabilization by class L amphipathic helical peptides: nucleation model of defect formation. Biophys. J. 81, 949–959 (2001).
Lasic, D.D. The mechanism of vesicle formation. Biochem. J. 256, 1–11 (1988).
Lasic, D.D. & Martin, F.J. On the mechanism of vesicle formation. J. Memb. Sci. 50, 215–222 (1990).
Watwe, R.M. & Bellare, J.R. Manufacture of liposomes—a review. Curr. Sci. 68, 715–724 (1995).
Winterhalter, M. & Lasic, D.D. Liposome stability and formation—experimental parameters and theories on the size distribution. Chem. Phys. Lipids 64, 35–43 (1993).
Armengol, X. & Estelrich, J. Physical stability of different liposome compositions obtained by extrusion method. J. Microencapsul. 12, 525–535 (1995).
Shingles, R. & McCarty, R.E. Production of membrane vesicles by extrusion: size distribution, enzyme activity, and orientation of plasma membrane and chloroplast inner-envelope membrane vesicles. Anal. Biochem. 229, 92–98 (1995).
Seantier, B. & Kasemo, B. Influence of mono- and divalent ions on the formation of supported phospholipid bilayers via vesicle adsorption. Langmuir 25, 5767–5772 (2009).
Kasemo, B. Biocompatibility of titanium implants: surface science aspects. J. Prosthet. Dent. 49, 832–837 (1983).
Kasemo, B. & Lausmaa, J. Aspects of surface physics on titanium implants. Swed. Dent. J. Suppl. 28, 19–36 (1985).
Greve, F. et al. Molecular design and characterization of the neuron-microelectrode array interface. Biomaterials 28, 5246–5258 (2007).
Reimhult, E., Hook, F. & Kasemo, B. Vesicle adsorption on SiO2 and TiO2: Dependence on vesicle size. J. Chem. Phys. 117, 7401–7404 (2002).
Modin, C. et al. QCM-D studies of attachment and differential spreading of pre-osteoblastic cells on Ta and Cr surfaces. Biomaterials 27, 1346–1354 (2006).
Ekeroth, J., Konradsson, P. & Höök, F. Bivalent-ion-mediated vesicle adsorption and controlled supported phospholipid bilayer formation on molecular phosphate and sulfate layers on gold. Langmuir 18, 7923–7929 (2002).
Rossetti, F.F., Textor, M. & Reviakine, I. Asymmetric distribution of phosphatidyl serine in supported phospholipid bilayers on titanium dioxide. Langmuir 22, 3467–3473 (2006).
Rossetti, F.F., Bally, M., Michel, R., Textor, M. & Reviakine, I. Interactions between titanium dioxide and phosphatidyl serine-containing liposomes: formation and patterning of supported phospholipid bilayers on the surface of a medically relevant material. Langmuir 21, 6443–6450 (2005).
Kunze, A., Sjovall, P., Kasemo, B. & Svedhem, S. In situ preparation and modification of supported lipid layers by lipid transfer from vesicles studied by QCM-D and TOF-SIMS. J. Am. Chem. Soc. 131, 2450–2451 (2009).
Jackman, J.A., Cho, N.J., Duran, R.S. & Frank, C.W. Interfacial binding dynamics of bee venom phospholipase A(2) investigated by dynamic light scattering and quartz crystal microbalance. Langmuir 26, 4103–4112 (2010).
Cho, N.J. et al. Quartz resonator signatures under Newtonian liquid loading for initial instrument check. J. Colloid Interface Sci. 315, 248–254 (2007).
Reimhult, E., Hook, F. & Kasemo, B. Temperature dependence of formation of a supported phospholipid bilayer from vesicles on SiO2. Phy. Rev. E 66 (2002).
Reimhult, E., Hook, F. & Kasemo, B. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: Influence of surface chemistry, vesicle size, temperature, and osmotic pressure. Langmuir 19, 1681–1691 (2003).
Zhdanov, V.P., Dimitrievski, K. & Kasemo, B. Adsorption and spontaneous rupture of vesicles composed of two types of lipids. Langmuir 22, 3477–3480 (2006).
Dimitrievski, K., Reimhult, E., Kasemo, B. & Zhdanov, V.P. Simulations of temperature dependence of the formation of a supported lipid bilayer via vesicle adsorption. Colloids Surf. B Biointerfaces 39, 77–86 (2004).
Dimitrievski, K. & Kasemo, B. Influence of lipid vesicle composition and surface charge density on vesicle adsorption events: a kinetic phase diagram. Langmuir 25, 8865–8869 (2009).
Sauerbrey, G. Verwendung von Schwingquarzen zur Wagung dunner Schichten und zur Mikrowagung. Z. Phys. 155, 206–222 (1959).
Voinova, M.V., Rodahl, M., Jonson, M. & Kasemo, B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: continuum mechanics approach. Phys. Scr. 59, 391–396 (1999).
Johannsmann, D., Reviakine, I. & Richter, R.P. Dissipation in films of adsorbed nanospheres studied by quartz crystal microbalance (QCM). Anal. Chem. 81, 8167–8176 (2009).
Richter, R.P. & Brisson, A.R. Following the formation of supported lipid bilayers on mica: a study combining AFM, QCM-D, and ellipsometry. Biophys. J. 88, 3422–3433 (2005).
Kasemo, B. & Lausmaa, J. Biomaterial and implant surfaces: on the role of cleanliness, contamination, and preparation procedures. J. Biomed. Mater. Res. 22 (A2 Suppl): 145–158 (1988).
Hook, F. et al. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloids Surf. B Biointerfaces 24, 155–170 (2002).
Mashaghi, A., Swann, M., Popplewell, J., Textor, M. & Reimhult, E. Optical anisotropy of supported lipid structures probed by waveguide spectroscopy and its application to study of supported lipid bilayer formation kinetics. Anal. Chem. 80, 3666–3676 (2008).
Richter, R.P., Maury, N. & Brisson, A.R. On the effect of the solid support on the interleaflet distribution of lipids in supported lipid bilayers. Langmuir 21, 299–304 (2005).
Stelzle, M. & Sackmann, E. Sensitive detection of protein adsorption to supported lipid bilayers by frequency-dependent capacitance measurements and microelectrophoresis. Biochim. Biophys. Acta. 981, 135–142 (1989).
Cho, N.J., Cho, S.J., Hardesty, J.O., Glenn, J.S. & Frank, C.W. Creation of lipid partitions by deposition of amphipathic viral peptides. Langmuir 23, 10855–10863 (2007).
Meyvis, T.K., De Smedt, S.C., Van Oostveldt, P. & Demeester, J. Fluorescence recovery after photobleaching: a versatile tool for mobility and interaction measurements in pharmaceutical research. Pharm. Res. 16, 1153–1162 (1999).
Bailey, L.E. et al. Multistep adsorption of perfluoropolyether hard-disk lubricants onto amorphous carbon substrates from solution. Langmuir 17, 8145–8155 (2001).
Bobardt, M.D. et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc. Natl Acad. Sci. USA 105, 5525–5530 (2008).
Cho, N.J. et al. The mechanism of an amphipathic α-helical peptide's antiviral activity involves size-dependent virus particle lysis. ACS Chem. Biol. 4, 1061–1067 (2009).
Han, X. et al. Supported bilayer lipid membrane arrays on photopatterned self-assembled monolayers. Chemistry 13, 7957–7964 (2007).
Purrucker, O. et al. Polymer-tethered membranes as quantitative models for the study of integrin-mediated cell adhesion. Soft Matter 3, 333–336 (2007).
Sklan, E.H. et al. A Rab-GAP TBC domain protein binds hepatitis C virus NS5A and mediates viral replication. J. Virol. 81, 11096–11105 (2007).
Yu, X. et al. Cryo-electron microscopy and three-dimensional reconstructions of hepatitis C virus particles. Virology 367, 126–134 (2007).
Acknowledgements
N.-J.C. is a recipient of an American Liver Foundation Postdoctoral Fellowship Award and a Global Roche Postdoctoral Fellowship. We wish to thank all the members of the Frank, Kasemo and Hook laboratories, who laid the foundation for future studies in the biomimetic sensor field.
Author information
Authors and Affiliations
Contributions
N.-J.C. and F.H. conceived and designed the protocol and wrote the paper; C.W.F. and B.K. contributed to data analysis and paper editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cho, NJ., Frank, C., Kasemo, B. et al. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat Protoc 5, 1096–1106 (2010). https://doi.org/10.1038/nprot.2010.65
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.65
This article is cited by
-
A drug-free cardiovascular stent functionalized with tailored collagen supports in-situ healing of vascular tissues
Nature Communications (2024)
-
Rectifying disorder of extracellular matrix to suppress urethral stricture by protein nanofilm-controlled drug delivery from urinary catheter
Nature Communications (2023)
-
Nanoarchitectonics-based model membrane platforms for probing membrane-disruptive interactions of odd-chain antimicrobial lipids
Nano Convergence (2022)
-
Sialylated glycan-modulated biomimetic ion nanochannels driven by carbohydrate–carbohydrate interactions
NPG Asia Materials (2022)
-
Ultrahigh resistance of hexagonal boron nitride to mineral scale formation
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.